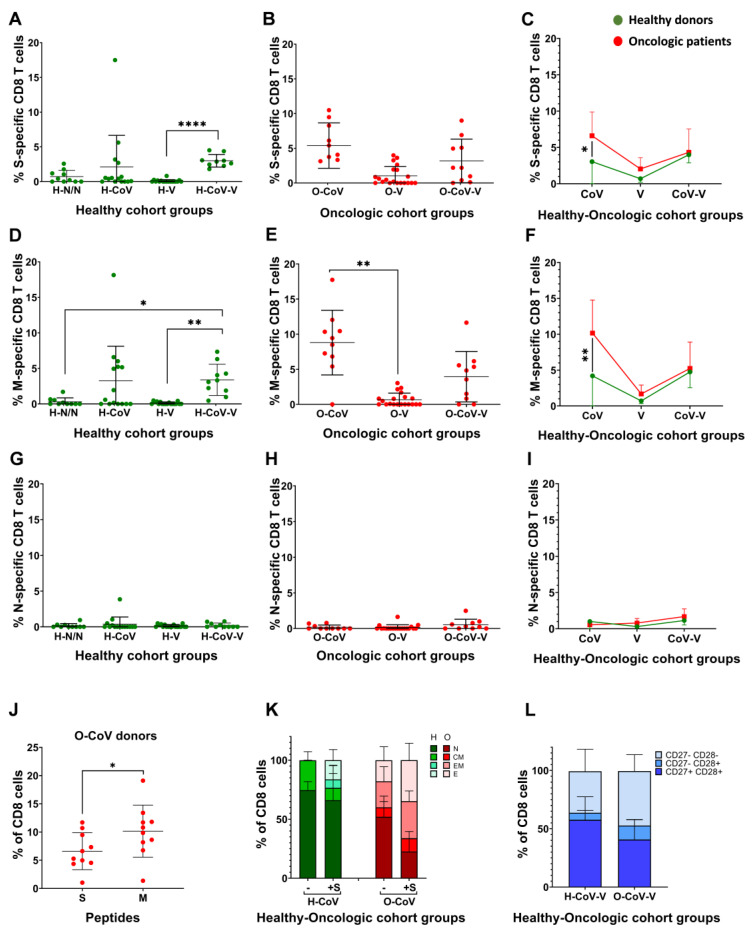
Figure 3.
CD8 T-cell responses to S, M and N peptides of SARS-CoV-2 proteins. Paired backgrounds from technical controls were removed from the data. (A–C) Percentage of S-specific CD8 T-cells in PBMCs stimulated with S-peptides in healthy donors and oncologic patients. (D–F) Percentage of S-specific CD8 T-cells in PBMCs stimulated with M-peptides in healthy donors and oncologic patients. (G–I) Percentage of S-specific CD8 T-cells in PBMCs stimulated with N-peptides in healthy donors and oncologic patients. (A,B,D,E,G,H) Significance was tested with Kruskal–Wallis tests, followed by Dunn’s test. (J) Percentage of activated CD8 T-cells after stimulation with S- or M-specific peptides in O-CoV donors. (C,F,I,J) U of Mann-Whitney was used to test for significance. (K) Relative percentages of CD8 T-cell differentiation phenotypes in the indicated groups of healthy donors and oncologic patients. Means and error bars (standard deviations) are shown. N, CM, EM and E, indicate naïve-stem cell (CD62L+ CD45RA+), central memory (CD62L+ CD45RAneg), effector memory (CD62Lneg CD45RAneg) and effector (CD62Lneg CD45RA+) phenotypes. Relevant statistical comparisons are detailed in Figure S8. (L) Relative percentages of CD8 T-cell differentiation phenotypes in the indicated groups of healthy donors and oncologic patients according to CD27neg CD28 expression profiles. CD27+ CD28+, CD27neg CD28+ and CD27+ CD28+ indicate poorly differentiated, intermediate differentiated and highly differentiated T-cell phenotypes. H-N/N—non-vaccinated, non-COVID-19 donors; H-CoV—healthy donors with previous COVID-19 infection; H-V—vaccinated healthy donor; H-CoV-V—vaccinated healthy donor with previous COVID-19; O-CoV—oncologic patient with previous COVID-19; O-V—vaccinated oncologic patients; O-CoV-V—vaccinated oncologic patients with previous COVID-19; *, ** and **** indicate significant (p < 0.05), very significant (p < 0.01) and very highly significant (p < 0.0001) differences, respectively.