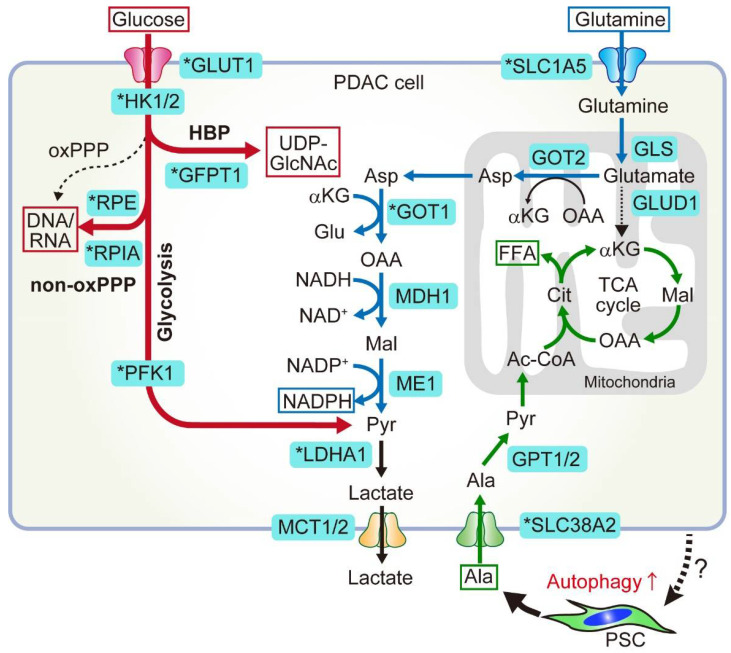
Figure 1.
Central carbon metabolism in pancreatic ductal adenocarcinoma (PDAC) is rewired in a cell-autonomous manner by oncogenic KRAS signaling and in a cell-extrinsic manner via interaction with host stromal cells. Key enzymes that are upregulated by oncogenic KRAS are indicated with an asterisk. (Glucose metabolism, shown in red) Glucose is preferentially used for anabolic processes (anabolic glucose metabolism) in PDAC. This is mediated by the upregulation of key enzymes involved in those processes. Glucose transporter 1 (GLUT1) upregulation increases the glucose uptake, which fuels glycolysis and branching anabolic pathways. These include the hexosamine biosynthesis pathway (HBP), which produces precursors for glycosylation, and the non-oxidative pentose phosphate pathway (non-oxPPP), which produces ribose for nucleotide biosynthesis. (Glutamine metabolism, shown in blue) Glutamine is a key substrate that is used to fuel the tricarboxylic acid (TCA) cycle and maintain redox balance in PDAC, which is mediated by a pathway distinct from all other types of cancers. (PSC-derived alanine, shown in green) As shown above, glucose and glutamine are primarily used for anabolic pathways and redox maintenance in PDAC. To supplement the TCA cycle, PDAC cells utilize alanine that is released from pancreatic stellate cells (PSCs) in the tumor microenvironment (TME). In response to stimuli from PDAC (unknown), PSCs secrete alanine through SLC1A4 in an autophagy-dependent manner. PDAC cells upregulate SLC38A2 to uptake PSC-derived alanine, which then fuels the TCA cycle and is used to produce macromolecules, including free fatty acids (FFA). Ac-CoA, acetyl coenzyme A; Asp, asparagine; αKG, α-ketoglutarate; Cit, citrate; Mal, malate; OAA, oxaloacetate. Figure is adapted from reference [41], the use of which is licensed under a Creative Commons Attribution 4.0 International license https://creativecommons.org/licenses/by/4.0/.