Abstract
Human aging, a natural process characterized by structural and physiological changes, leads to alterations of homeostatic mechanisms, decline of biological functions, and subsequently, the organism becomes vulnerable to external stress or damage. In fact, the elderly population is prone to develop diseases due to deterioration of physiological and biological systems. With aging, the production of reactive oxygen species (ROS) increases, and this causes lipid, protein, and DNA damage, leading to cellular dysfunction and altered cellular processes. Indeed, oxidative stress plays a key role in the pathogenesis of several chronic disorders, including hepatic diseases, such as non-alcoholic fatty liver disease (NAFLD). NAFLD, the most common liver disorder in the Western world, is characterized by intrahepatic lipid accumulation; is highly prevalent in the aging population; and is closely associated with obesity, insulin resistance, hypertension, and dyslipidemia. Among the risk factors involved in the pathogenesis of NAFLD, the dysbiotic gut microbiota plays an essential role, leading to low-grade chronic inflammation, oxidative stress, and production of various toxic metabolites. The intestinal microbiota is a dynamic ecosystem of microbes involved in the maintenance of physiological homeostasis; the alteration of its composition and function, during aging, is implicated in different liver diseases. Therefore, gut microbiota restoration might be a complementary approach for treating NAFLD. The administration of probiotics, which can relieve oxidative stress and elicit several anti-aging properties, could be a strategy to modify the composition and restore a healthy gut microbiota. Indeed, probiotics could represent a valid supplement to prevent and/or help treating some diseases, such as NAFLD, thus improving the already available pharmacological intervention. Moreover, in aging, intervention of prebiotics and fecal microbiota transplantation, as well as probiotics, will provide novel therapeutic approaches. However, the relevant research is limited, and several scientific research works need to be done in the near future to confirm their efficacy.
Keywords: liver, age-related disease, NAFLD, microbiota, therapeutic strategies
1. Introduction
The so-called gut microbiota (GM) is constituted by numerous different populations of microorganisms (bacteria, archaea, fungi, and viruses) that reside in the gastrointestinal tract of mammals. In recent years, a significant interest in the intestinal microbiota has spread, as it is considered one of the key factors contributing to the maintenance of physiological intestinal homeostasis, the protection against pathogens, and the modulation of the immune system. All these important functions make the GM a fundamental system able to regulate the host’s health [1,2]. Many researches on GM composition, conducted both in animals and humans, have highlighted its involvement in the onset and progression of several disorders, including neurodegenerative; cardiovascular; gastrointestinal; and metabolic diseases, such as obesity, type 2 diabetes, and non-alcoholic fatty liver disease (NAFLD) [3].
The progressive degeneration of the tissues, with consequent alteration of organs’ structure and function, and the loss of homeostasis, make the elderly people more prone to develop diseases [4,5,6]. During aging, it is widely reported that the increased imbalance between reactive oxygen species (ROS) production and antioxidant enzymes expression leads to the onset of oxidative stress (OS), with consequent damage to proteins, DNA, and cellular organelles [7]. Specifically, in the gut, OS, together with a sedentary lifestyle, changes in diet, and administration of drugs, causes GM dysbiosis, which contributes to the increase in intestinal permeability, resulting in the release of bacteria, endotoxins, and pro-oxidants into the systemic circulation. Ultimately, all these factors contribute to the development of hepatic diseases, such as NAFLD [8]. Currently, NAFLD is considered the most common chronic liver disease in the Western world and it is characterized by an excessive intrahepatic fat accumulation, and strongly associated with obesity, hypertension, and insulin resistance [9]. The pathogenesis of NAFLD is not completely understood, but the most accredited hypothesis is the interaction among environmental factors (such as a hypercaloric diet), GM changes, sedentary lifestyle, and genetic predisposition [10]. Over time, NAFLD can become non-alcoholic steatohepatitis (NASH), and eventually progress into fibrosis, cirrhosis, and hepatocellular carcinoma [11]. In order to block the progression of NAFLD, thus improving the elderly’s health, the prevention of the disease is important. The use of probiotics, which are alive microorganisms with numerous health benefits, could be a valid strategy, thanks to their ability to restore the GM and relieve oxidative stress [12].
This review aims to underline the possible factors causing GM dysbiosis and intestinal permeability disruption in elderly people, focusing above all on OS, with particular attention to the association between an altered GM and the development of NAFLD. We also discuss the NAFLD-associated GM signatures and the use of probiotics as a potential therapeutic strategy to restore GM to a healthy condition and counteract NAFLD progression.
2. Non-Alcoholic Fatty Liver Disease and Non-Alcoholic Steatohepatitis
Non-alcoholic fatty liver disease is an umbrella term including simple steatosis or non-alcoholic fatty liver (NAFL) and its progression into non-alcoholic steatohepatitis. NAFLD is the hepatic manifestation of the “metabolic syndrome”, which also comprises dyslipidemia, hypertension, insulin resistance, and diabetes [13,14]. Recently, the term NAFDL has been proposed to be replaced by the more generic definition of metabolic-associated fatty liver disease (MAFLD), even though, in general, the traditional nomenclature is still preferred by the majority of experts, mainly because many clinical trials are currently specifically targeting NASH [15]. About a quarter of the world population suffers from NAFLD [16], with rates exceeding 43% in patients with metabolic syndrome [17]. Progression into NASH has been observed in about 10% of patients suffering from NAFLD, and more commonly in patients suffering from diabetes (37.7%), who also present the highest prevalence rate of NAFL (55.5%) [18]. In NASH, the hepatic fat deposition is accompanied by an increased free fatty acid oxidation and mitochondrial dysfunction, leading to a chronic inflammatory state, which in turn can lead to high risk of fibrosis, cirrhosis, and hepatocellular carcinoma development [11,19]. Traditionally, two main “hits” were believed to be involved in NAFLD pathogenesis, being the first intrahepatic fat accumulation triggered by a sedentary lifestyle, bad nutritional habits, and insulin resistance [20], and the second a lipid-induced over-production of ROS [21]. The two-hit hypothesis, by a general consensus, is now considered too simplistic and a “multiple-hit hypothesis” has been proposed instead [11]. The multiple-hit hypothesis has been described as an “integrated response” of the organism to the combination of hypercaloric nutrition and sedentary lifestyle in a genetically predisposed host, leading to metabolic syndrome and obesity [22]. These events are accompanied by insulin resistance in the muscle in response to the increased levels of circulating free fatty acids, leading to an increase in hepatic de novo lipogenesis (DNL) and an imbalance in adipose tissue lipolysis, resulting in higher levels of circulating fatty acids conveyed to the liver [22]. Insulin resistance also contributes to the release of adipokines and inflammatory cytokines from the adipose tissue [23]. Aging affects the process of de novo lipogenesis (DNL) mostly through changes in systemic mediators such as insulin and leptin; in fact, aging is an insulin- and leptin-resistant state [24]. Many factors contribute to insulin resistance in aging, including an increase in body adiposity and visceral fat, increased adipose tissue inflammation, an increase in circulating cytokines, sedentary life style, and changes in growth hormone/insulin-like-growth-factor I (GH-IGF) axis [25]. Insulin resistance has been shown to induce an increase in the percent contribution of DNL to hepatic lipid accumulation [26]. Transcription factors sterol regulatory element-binding protein (SREBP)-1c and carbohydrate-responsive element-binding protein (ChREBP)-1, driven by insulin and glucose respectively, play a crucial role in stimulating DNL in the hepatocytes through an increase in the transcription of rate-limiting DNL enzymes such as fatty acid synthase (FAS), stearoyl-CoA desaturase-1 (SCD1), and acyl coA carboxylase (ACC) [27]. Similarly to insulin resistance, in aging also serum leptin levels are increased, along with a paradoxical lack of effects due to multiple causes, including receptor desensitization, mutations in the genes encoding leptin and its receptors as well as proteins involved in self-regulation of leptin synthesis, and changes in blood–brain barrier permeability [28,29]. Leptin serves as the “satiety signal”, acting primarily at the level of the hypothalamus to decrease appetite; so, reduced leptin levels or leptin resistance result in a higher food intake [30]. In addition to its central role, leptin may have a direct action on DNL; receptors for leptin have also been found in peripheral tissues including liver [31], so changes in leptin signaling may also result in direct DNL enzymes positive modulation [24]. In the liver, excessive fat accumulation leads to lipotoxicity, a condition promoting oxidative stress and affecting mitochondrial and endoplasmic reticulum physiological functions [32]. Altogether, these processes lead to hepatic chronic inflammation accompanied by cell death, hepatic stellate cell (HSC) activation, and fibrosis. However, the original assumption that steatosis always precedes inflammation is not always correct; in fact, NASH can also be the initial hepatic injury: it is the timing and the combination of insults that determine whether steatosis or NASH will occur first [33]. Recently, a deficit of lipophagy has been identified as a further contributor to lipid overaccumulation in NAFLD pathogenesis [34]. Lipophagy is a highly regulated step process that consists of: (1) protein-mediated sequestration of lipid droplets within cytosolic vesicles and formation of a phagosome; (2) transport of a phagosome to a lysosome and formation of the autophagolysosome; and (3) lipid degradation by lysosomal lipases [35]. Many proteins are involved in this process. The cargo adapter p62 is essential as it connects the lipidic cargo with autophagosomes; elevated P62 levels usually are a marker or decreased autophagy. LC3-II, a protein that targets to the elongated autophagosome membrane, is degraded by lysosomal proteases; therefore, the increase in LC3-II indicates its impaired turnover [36]. Both P62 and LC3-II proteins were found to accumulate in high-fat diet-fed C57BL/6J male mice and high-fat/high-glucose cultured Huh7 cells [35]. In addition, in NAFLD patients, lipid droplet-loaded lysosomes and P62/sequestosome (SQSTM)1 clusters were associated with NAFLD activity score (NAS) and fibrosis stage, respectively, as well as expression levels of lysosomal genes and autophagy-related genes, showing that impaired autophagy is associated with features of advanced disease [35].
3. Gut Microbiota and Oxidative Stress
The human microbiota consists of a wide range of microorganisms that reside in different parts of the body, including the skin and the gastrointestinal, genitourinary, and respiratory tracts [37]. In addition to these body districts, the urethra and the mammary glands have their own microbes [38]. The GM is a complex and dynamic ecosystem of trillions of commensal microorganisms, including different communities of bacteria and some members of archaea, fungi, and viruses, which live in the gastrointestinal tract and give rise to a mutual relationship with the host [39,40]. The colonization of the gastrointestinal tract by bacteria begins in utero, via the placenta and the mother’s amniotic fluid [41], while after birth, the mode of delivery (natural or cesarean), feeding (breastfeeding or artificial), ingestion of antibiotics or probiotics during the early days of life, genetics, and environmental factors influence the composition of GM [39,42]. The Bifidobacterium mainly dominates the microbiota profile of infants, which can continually change during the first 3 years of life [43] according to a variety of factors, such as nutrition, geographic distribution [44], genetic background, and immunological stimuli [45]. After 3 years of age, the GM acquires a more complex adult pattern that is relatively stable throughout adulthood [37]. Namely, the GM of healthy adults is composed of anaerobic bacteria, most of which (more than 90%) belong to the phyla of Bacteroidetes (Bacteroides, Prevotella, and Porphyromonas) and Firmicutes (Clostridia), followed by a small percentage (1–8%) of Actinobacteria (Bifidobacterium), Proteobacteria, and Verrucomicrobia [46,47]. The GM is necessary for the human’s health; indeed, it can modulate innate and adaptive immune responses, regulate cellular growth, and preserve epithelial barrier function [48]. Furthermore, the GM is also involved in glucose and lipid metabolism, energy balance, detoxification, vitamin K synthesis, and production of short-chain fatty acids (SCFAs; acetate, propionate, and butyrate) [45,47].
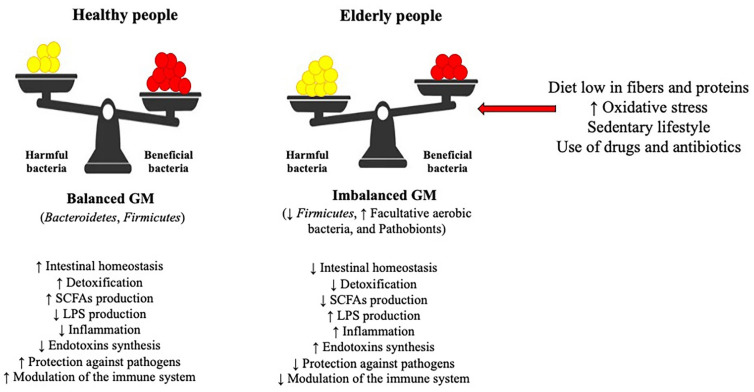
On the whole, based on its composition, the GM is described as an organ with a high degree of variability and heterogeneity, able to change and adapt to the needs of the host’s human body [37]. As a result, each person’s GM has a unique composition that differs from others [49]. Nevertheless, the GM composition changes drastically as people age due to various factors, including lifestyle, dietary habits, stress, use of antibiotics and drugs, and environmental stimuli [39,50] (Figure 1). This event results in damage and loss of the intestinal homeostasis [51], and thus, in the elderly, the GM acquires specific features such as fewer beneficial bacteria, changes in dominant gut species (reduction of Firmicutes and increase in facultative aerobic bacteria), and proliferation of pathobionts proliferation (streptococci, staphylococci, enterobacteria, and enterococci), which are responsible for the onset in the gut of an inflammatory state in the gut [37,52,53]. Further, the dysbiotic microbiota is no longer able to perform its primary beneficial functions, thus leading to the production of toxic metabolites and to inflammation, which in turn cause the development of a variety of metabolic diseases, such as hypercholesterolemia, diabetes, obesity, NAFLD, and its progression into NASH [54,55].
Figure 1.
Effects of an altered gut microbiota (GM) in elderly people. A diet low in fibers and proteins, an increase in oxidative stress, a sedentary lifestyle, and an intake of drugs and antibiotics lead to an alteration of the intestinal microbiota, with a reduced production of SCFAs and an increase in LPS, resulting in gut inflammation and development of metabolic diseases. Abbreviations: SCFAs: shot-chain fatty acids; LPS: lipopolysaccharides.
Since elderly people have difficulties in swelling and chewing, in association with a decreased digestive motility [56], nutrition plays a key role in changing the GM profile [57]. A diet lacking in fibers and proteins, for example, as well as vitamin D and calcium deficiency, can alter the composition of GM, [58,59]. Moreover, the consumption of plant-based proteins, animal-based proteins, inulin, olive oil, and omega-3 polyunsaturated fatty acids (PUFA) can also modulate GM [60]. Besides diet, GM dysbiosis can as well be caused by oxidative stress and treatment with drugs aimed at targeting human cells rather than microorganisms, such as antidiabetics (metformin), proton pump inhibitors (PPIs), nonsteroidal anti-inflammatory drugs (NSAIDs), and atypical antipsychotics (AAPs) [61,62].
The accumulation of ROS produced by cellular metabolic and respiratory processes is recognized as one of the causes that promotes aging [63]. Namely, ROS and reactive nitrogen species (RNS) are essential for cellular proliferation and differentiation, cytokines release, metabolism, and immune response. They are naturally produced by the organism’s cells at low levels [7]. Under physiological conditions, the organism has several antioxidative defense mechanisms, including enzymes (catalase, glutathione peroxidase, and superoxide dismutase) and antioxidants (such as vitamin C, vitamin E, uric acid, carotenoids, and flavonoids), which can protect it against oxidant species; instead, as people get older, there is a cellular imbalance between these defenses and ROS generation, in favor of oxidants, resulting in OS [7,64]. OS causes molecular and cellular damage, particularly to proteins, lipids, DNA, and organelles [7,65], thus contributing to uncontrolled proliferation, inflammation, and cell death through apoptosis [66].
During aging, the alteration of cellular macromolecules, as well as the dysfunction at the level of mitochondria, which represents the primary source of energy, and the subsequent further production of ROS, eventually lead to the onset of age-related disorders [64].
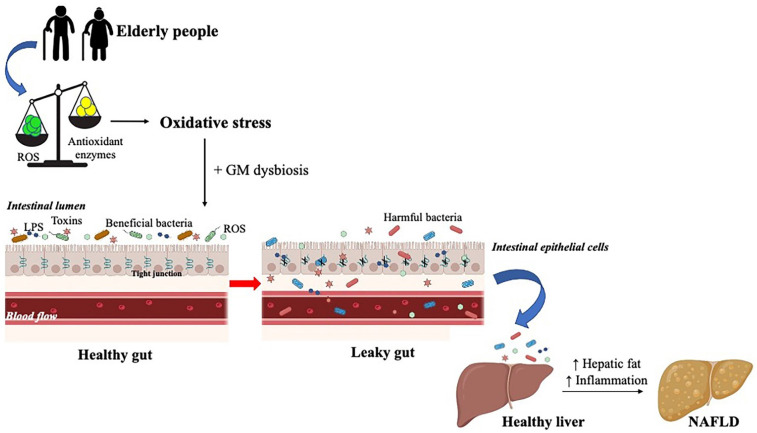
Specifically, at the intestinal level, the continuous exposure of the mucosa to oxidants derived from diet and bacteria results in excessive production of ROS and the consequent onset of oxidative stress [66]. OS may disrupt colonic epithelial tight junctions, subsequently increasing the intestinal mucosa permeability, thus leading to a phenomenon known as “leaky gut” syndrome. This condition is characterized by the translocation of pro-oxidants and antigens, such as lipopolysaccharides (LPS), bacteria, and their endotoxins into the systemic circulation, where they reach various target organs [40,46], resulting in several pathological conditions, including metabolic disorders and infectious and systemic diseases (such as cardiac, neurodegenerative, and neoplastic) [40,67].
As mentioned before, whereas the composition of GM seems to be directly associated with ROS production in the intestine [68], other authors [60], conversely revealed that the abundance and composition of the GM may influence the intestinal production of ROS. In fact, the consumption of probiotic bacteria and antioxidant nutrients able to change GM may lower ROS production by inhibiting pro-oxidant enzymes and stimulating antioxidant enzymes and related pathways [60]. Furthermore, the GM itself can produce antioxidant molecules (glutathione, butyrate, and folate) able to protect the gut from toxins and ROS [69].
Finally, given that an imbalance between oxygen species generation and antioxidant defenses causes intestinal damage, an excessive amount of ROS and RNS leads to an elevated cellular oxidative stress, contributing to the GM dysbiosis, which favors several gastrointestinal conditions, such as inflammation and metabolic disorders, like NAFLD [60]. In fact, the alteration of the intestinal microbiota is an important factor that contributes to the pathogenesis of NAFLD and its progression into NASH [54]. In particular, dysbiosis and oxidative stress lead to a dysregulation of intestinal permeability, resulting in the release of endotoxins, and microbiota metabolites, derived from saccharolytic and proteolytic fermentation, at the level of the liver, with a consequent increase in the accumulation of hepatic fat and inflammation [8], typical signs of this disease. Over time, if these conditions persist, NAFLD progresses into NASH, the more severe form, characterized by hepatocellular injury, chronic inflammation, and fibrosis [70] (Figure 2).
Figure 2.
”Leaky gut” syndrome contributes to the onset of NAFLD. In the elderly, the imbalance between the formation of ROS and the presence of antioxidant enzymes leads to oxidative stress, which, at the intestinal level contributes to gut microbiota (GM) dysbiosis. These two conditions disrupt the tight junctions of the epithelial cells, resulting in the translocation of endotoxins, lipopolysaccharides (LPS), harmful bacteria, and ROS from the intestinal lumen into the blood circulation where they are taken up by the liver and cause an increase in lipogenesis and inflammation, finally resulting in the development of this hepatic disease.
4. Gut Microbiota and NAFLD Development in Animal Models
In the last decades, fecal transplantation experiments in mice have provided a growing body of evidence about a causal role between GM alterations and NAFLD/NASH development [71,72]. GM was studied in various animal models of NAFLD, and its alteration was found to be associated with NAFLD genesis and progression. Adult germ-free mice fed with a regular diet, when exposed to a microbiota harvested from conventionally raised animals, showed a 60% increase in body fat content and the insurgence of insulin resistance, possibly due to the increase in the absorption of monosaccharides from the gut lumen, resulting in the induction of hepatic de novo lipogenesis [73]. Similarly, wild-type germ-free mice fed with a Western-style, high-fat, sugar-rich diet, are less prone to develop steatosis when compared to animals raised in conventional conditions [74,75]. On the contrary, steatosis develops regularly in germ-free knockout (KO) mice lacking fasting-induced adipose factor (FIAF), a circulating lipoprotein lipase inhibitor normally suppressed by GM, suggesting that FIAF is a mediator of microbial-regulated energy storage [74]. Recognizing the role of GM in the development of NAFLD also implies the concept that NAFLD is potentially a transmissible process [76]. In fact, germ-free mice colonized with the cecal content collected from donors either responders or non-responders to a high-fat diet developed symptoms comparable to the respective donor when fed with the same diet. In other words, germ-free mice receiving intestinal microbiota from responder mice developed macrosteatosis and hyperglycemia; differently, mice receiving intestinal microbiota from non-responder mice do not develop NAFLD when fed with a high-fat diet [77]. A similar effect was seen in mice colonized with human GM from healthy individuals or NAFLD patients: mice fed with a high-fat diet developed more severe NAFLD symptoms when receiving the microbiota from NAFLD patients and vice-versa [78]. In a more recent work, quercetin was administered to donor mice fed with a high-fat diet to modulate the microbiota composition; the transplantation of microbiota from quercetin-treated donors in germ-free mice resulted in a protective phenotype against diet-induced NAFLD [79]. Further, in mice fed with a Western diet, a worsening of NASH symptoms was associated with the depletion of G protein-coupled chemokine receptor CX3CR1; the depletion of GM using broad-spectrum antibiotics was also found to protect mice from diet-induced NASH, similarly to what was demonstrated in germ-free mice [80].
The methionine-choline deficient (MCD) diet is another well-established animal model of NAFLD. In this model, choline deficiency affects triglyceride export via very low-density lipoproteins (VLDLs), resulting in hepatic steatosis; in addition, the lack of methionine impairs glutathione synthesis, causing a significant increase in oxidative injury [14]. The MCD diet-induced NAFLD is characterized by hepatic ballooning, marked oxidative stress, chronic inflammation, and fibrosis, without development of hyperglycemia, dyslipidemia, and insulin resistance; therefore, it is more suitable for the study of inflammation and fibrosis [81]. In mice, the administration of MCD diet induces persistent alterations in the GM and impairment of the intestinal barrier [82]. However, unexpectedly, in MCD mice the treatment with broad-spectrum antibiotics, aimed to deplete the microbiota, does not produce the same effect seen in germ-free or antibiotic-treated mice fed with a Western diet, resulting in the aggravation of steatosis, inflammation, a higher histopathological NAFLD activity score (NAS), and a significantly higher liver-to-body weight ratio [83]. In contrast, the microbiota modulation via probiotics has shown beneficial effects both in the high-fat NAFLD model and in the MCD-induced NASH [84], suggesting that in the MCD model, the microbiota preserves its protective activity, which is lost in high-fat and Western diet models of NAFLD. These last works suggest that the microbiota should be seen as both a potential therapeutic agent and a drug target for the treatment of NAFLD.
An alternative NAFLD model consists of the supplementation of high doses of fructose in a regular diet [14]. Interestingly, high fructose supplementation does not necessarily result in body weight gain, but indeed in the increase in liver weight/body weight ratio [75], supporting the newly acquired notion that diet-induced liver steatosis does not necessarily precede body weight gain [85]. Fructose at high doses has been found to be associated with microbiota overgrowth and increased intestinal permeability, leading to an endotoxin-dependent activation of hepatic Kupffer cells. In fact, the suppression of endotoxin-mediated activation of Kupffer cells in toll-like receptor (TLR)-4 mutant mice resulted in the reduction of hepatic triglyceride accumulation by approximately 40% in comparison with fructose-fed wild-type mice [86]. Fructose-induced steatosis is also absent in germ-free mice, confirming that bacterial products such as LPS are required to induce liver steatosis and clearly indicating that the gut microbiota is involved in the pathogenesis of experimental fatty liver disease [75].
Several hypotheses have been formulated as to how the GM may contribute to NAFLD development and progression into NASH. As previously mentioned, they include increased intestinal permeability, leading to an increased absorption by the host of microbially produced toxins and metabolites, such as LPS, trimethylamine N-oxide (TMAO), choline, and ethanol, which trigger inflammation and affect immunity [71]. Infiltrating immune cells such as monocyte-derived macrophages and neutrophil granulocytes, two mediators of the hepatic inflammation during NASH, seem to have a relevant role in the microbiota-mediated worsening of NAFLD; in fact, chemokine receptor antagonists, by inhibiting monocyte recruitment, reduce hepatocyte ballooning, fibrosis, and inflammation in both the Western diet and the MCD diet models [87]. Infiltrating immune cells express high levels of pathogen recognition receptors (PRRs), including the NLR inflammasome family members, designated to recognize toxins released by the microbiota that reach the liver via the portal circulation [88]. Interestingly, in NLRP3 and NLRP6 inflammasome-deficient mice, an unfavorable intestinal microbiota has been linked to a loss of intestinal barrier integrity and increased translocation of toxins of microbial origin into the liver, where they activate hepatic inflammation [89]. These data indicate that translocation of bacterial products from the gut into the liver is part of a highly regulated series of complex interactions among the gut, its microbiota, and the liver, often referred to as the gut–liver axis, and contributing to liver fat accumulation and inflammation in NASH [90].
5. Changes in Gut Microbiota in Animal Models of NAFLD
Many preclinical studies have tried to associate specific alterations in GM composition, often referred to as a microbial signature, with NAFLD and NASH development. Prolonged (80 weeks) high-fat diet feeding in mice was associated with an increase in the relative abundance of the Firmicutes phylum with respect to the Bacterioidetes; at the genus level, an increase in the abundance of Adercreutzia (Actinobacteria), Coprococcus (Firmicutes), Dorea (Firmicutes), and Ruminococcus (Firmicutes) was observed in mice fed with a high-fat diet in comparison with the low-fat diet group [91]. In germ-free mice colonized with the microbiota from responder and non-responder mice to high-fat diet, NAFLD was positively associated with Barnesiella and Roseburia (from the Bacteroidetes and Firmicutes genera, respectively); after 16 weeks of high-fat diet administration, an increase in Barnesiella and Allobaculum and a decrease in Lactobacilli were observed. In general, the Firmicutes phylum was more represented in mice developing NAFLD [77]. Overall, the increase in Firmicutes/Bacteroidetes has been associated with NAFLD progression, even though there is not a complete consensus. In this last regard, in another work, Firmicutes and Verrucomicrobiota phyla were instead found to be more represented in mice not developing NAFLD and, at the genus level, Bacteroidia and Flavobacteriia were increased in mice developing NAFLD [79]. The administration of VSL#3, a high-concentration mixture of Bifidobacteria, Lactobacilli, and Streptococcus thermophilus improved liver histology, reduced hepatic total fatty acid content, and decreased serum alanine aminotransferase levels in mice fed with high-fat diet. The histological and biochemical improvement were associated with lower levels of two nuclear factors regulated by tumor necrosis factor (TNF): Jun N-terminal kinase (JNK) and nuclear factor B (NF-B), both involved in the development of insulin resistance [84].
In mice fed with the MCD diet for 2 and 4 weeks, the phylum of Tenericutes was more abundant compared with that of the respective control groups, while Verrucomicrobia were consistently less abundant. After 2 weeks of MCD diet, a significantly higher abundance of Firmicutes and a significantly reduced content of Proteobacteria were seen; at 4 weeks, a decrease in Actinobacteria was observed. At the family level, Rikenellaceae, Desulfovibrionaceae, and Verrucomicrobiaceae were persistently reduced in the MCD group when compared with the 4-week control group [82]. After 8 weeks, MCD feeding resulted in a strong overall decrease of the microbiota diversity and in a reduction in the potentially probiotic Lactobacillus, as well as Akkermansia, and an increase in the Ruminococus, which has been linked to liver fibrosis [83].
6. Association between Gut Microbiota and NAFLD Development in Humans
Several large human studies have investigated a microbial signature possibly predicting the risk of progression from simple steatosis toward more advanced disease stages [76]; however, a certain level of discrepancy was found among studies, with divergent results concerning phylum, family, genus, and species. The phyla of Firmicutes and Bacteroidetes are the most represented in the gut microbiome; consequently, many animal and human studies focused on the relative abundance of these two groups. Similarly to what had been found in animal studies [77,91,92], it was originally proposed that an increase in the Firmicutes-to-Bacteroidetes ratio was associated with a higher energy harvest and more severe NAFLD manifestations in obese individuals [93]; however, this notion was challenged by more recent findings [94,95,96]. Specifically, in NAFLD patients, Firmicutes were found to be increased in studies by Del Chierico (2017) and Loomba (2017) [97,98], decreased in studies by Wang [99] and Zhu [94], and unaltered in those by Raman (2013) and Alferink [96,100]. Bacteroidetes were more represented in NAFLD patients in the studies by Wang [99] and Zhu [94], decreased in the studies by Del Chierico [97] and Shen [95], and unaltered in the studies from Alferink [96]. It has been proposed that using higher phylogenetic levels (i.e., phylum) to distinguish disease states naturally leads to discrepancies; therefore, the studies should focus on lower levels, such as the genus [100]; however, discrepancies have also been found when considering the genus level, with regard to Prevotella, Oscillibacter, Bifidobacterium, Blautia, Lactobacillus, and Roseburia [72]. These discrepancies may originate from the fact that NAFLD is heterogeneous by nature, and the studies often include patients at different stages of disease severity, with compensated or decompensated cirrhosis [72].
Nonetheless, concordant changes were found in patients with NAFLD and NASH, in comparison with healthy individuals. Indeed, the phylum of Proteobacteria was increased [95,98,100]; at the family level, Enterobacteriaceae were increased [94,95], while Rikenellaceae [94,97] and Ruminococcaceae [95,100] were decreased; the genera Faecalibacterium [94], Coprococcus [94,99], and Anaerosporobacter [99] were also decreased, while Dorea was increased [97,100]. An increase in the genera Escherichia and Peptoniphilus was specific to NAFLD patients without NASH [94,97], as well as a decrease in Prevotella [101].
7. Probiotics
According to the Food and Agriculture Organization of the United Nations (FAO)/World Health Organization (WHO), probiotics are defined as “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host” [102]. To be used as probiotics, microorganisms must have certain characteristics. They must be alive, safe, non-pathogenic bacteria, able to cross the gut intestinal tract and survive both in acidic (stomach) and basic (duodenum) pH, as well as be resistant to bile, hydrochloric acid, and pancreatic juice [103]. Furthermore, they should be of human origin, isolated from the mouth, gastrointestinal tract, or feces, and belong to a healthy GM. However, probiotic bacteria from the Lactobacillus and Bifidobacterium genera, as well as other microorganisms, can be also isolated from fermented milk and related products, such as cheese, and yogurt, as well as from traditional drinks (Yosa, Bosa, Pozol, and Togwa) [104]. In addition to these important features, it is essential that probiotics are endowed with an antimicrobial activity against pathogenic bacteria and a reduced intestinal permeability, which allows them to colonize the gut, as well as the ability to stimulate the immune system, by sending signals to gut immune cells, produce lactic acid, and influence intestinal metabolism [104].
It is known that probiotics have beneficial effects for both humans and animals, as they can promote gastrointestinal tract motility and control the intestinal microbiota, improve lactose tolerance, and lower cholesterol levels [105]. They can also favor the proliferation and differentiation of epithelial cells, and reinforce the intestinal barrier [106]. Furthermore, having a therapeutic role, they can confer benefits to the immune, nervous, and gastrointestinal systems, and prevent some diseases, including obesity, diabetes, cardiovascular, liver, and metabolic disorders, as well as cancer and allergies [107,108]. Additionally, they are a helpful solution to counteract other clinical conditions, such as diarrhea, gastroenteritis, Crohn’s disease, female urogenital infections, and alleviate symptoms due to lactose intolerance [104,109] (Table 1).
Table 1.
Use of probiotics in several disorders.
| Disease | Probiotic | Reference |
|---|---|---|
| Acute diarrhea | Lactobacillus rhamnosus GG | [110] |
| Allergic rhinitis | Lactobacillus acidophilus L-92 | [111] |
| Antibiotic-associated diarrhea | Saccharomyces boulardii | [112] |
| Asthma | Enterococcus faecalis FK-23 | [113] |
| Atopic dermatitis | Lactobacillus paracasei, and Lactobacillus fermentum | [114] |
| Atopic eczema | Mixture (Bifidobacterium bifidum, Bifidobacterium lactis, and Lactobacillus acidophilus) | [115] |
| Bacterial vaginosis | Saccharomyces cerevisiae Lactobacillus acidophilus, Lactobacillus rhamnosus GR-1, and Lactobacillus fermentum RC-14 | [116] [117] |
| Cardiovascular disorder | Lactobacillus rhamnosus GG | [118] |
| Chronic diarrhea | Lactobacillus plantarum CCFM1143 | [119] |
| Colon cancer | Lactobacillus rhamnosus Lactobacillus acidophilus, Mixture (Bifidobacteria bifidum, and Bifidobacteria infantum) | [120] [121] |
| Crohn’s disease | Escherichia coli Nissle 1917 Saccharomyces boulardii | [122] [123] |
| Diabetes | Lactobacillus acidophilus | [124] |
| Diarrhea | Bifidobacterium bifidum FSDJN705, and Bifidobacterium breve FHNFQ23M3 | [125] |
| Gastroenteritis | Lactobacillus F19 | [126] |
| Hypercholesterolemia | Enterococcus faecium M-74 | [127] |
| Lactose intolerance | Lactobacillus acidophilus DDS-1 | [128] |
| Liver disorder | Escherichia coli Nissle VSL#3 | [129] [130] |
| Metabolic disorder | Bifidobacterium adolescentis Z25 | [131] |
| Obesity | Lactobacillus plantarum K50 | [132] |
| Urinary tract infections | Lactobacillus rhamnosus GR-a and Lactobacillus reuteri RC-14 | [133] |
Interestingly, probiotics, by possessing anti-aging properties, could also favor longevity. In fact, they can be used to activate antioxidant and immunomodulatory pathways, as well as to prevent some typical signs of aging, such as hair loss and skin wrinkles, and to improve skin elasticity [134,135]. Wen-Yang Lin (2022) [12], by administering a mixture of probiotics (Bifidobacterium animalis subsp. infantis BLI-02, Bifidobacterium breve Bv889, Bifidobacterium bifidum VDD088, Bifidobacterium animalis subsp. lactis CP-9, and Lactobacillus plantarum PL-02) to aged mice, demonstrated their antioxidant property, resulting in positive modulation of GM and SCFAs synthesis [12]. Moreover, several clinical trials reported the effect of probiotics supplementation on the GM in elderly people [136,137,138,139]. For instance, two randomized, double-blind, placebo-controlled studies showed that Bifidobacterium longum Bar33 and Lactobacillus helveticus Bar13 reduce opportunistic pathogens (Clostridium cluster XI, Clostridium difficile and Clostridium perfringens, Enterococcus faecium and Campylobacter), while supplementation with Bifidobacterium longum 46 and B. longum 2C increases the number of Bifidobacterium catenulatum, Bifidobacterium bifidum, and Bifidobacterium breve [136,137].
Probiotics exert their health benefits through different mechanisms of action: They can compete with pathogenic bacteria for nutrients and adhesion sites on the intestinal mucosa, inhibit the production of bacterial toxins, fortify the epithelial barrier, and possess antimicrobial properties, such as the ability to produce antimicrobial substances (like bacteriocins and SCFAs), through which they can inhibit the growth of pathogens and restrict their adhesion and access across the barrier. Finally, probiotics can also act as immunomodulators, by reducing proinflammatory cytokines secretion [such as interferon gamma (IFNγ), TNFα, and interleukin 12 (IL-12)] and promoting the expression of anti-inflammatory cytokines (such as IL-10), as well as epithelial cells and T lymphocytes proliferation and differentiation [140,141,142].
In addition to the positive effects mentioned above, supplementation with probiotics can also modulate the GM in humans and animals [107]. In the presence of dysbiosis, probiotics species can restore the correct balance of the gut microbial composition and repress pathogens, by eliciting the production of β-defensin and IgA; moreover, they can favor the expression of anti-inflammatory molecules and improve the integrity of the intestinal barrier, by promoting the production of mucin and preventing the disruption of tight junctions [40,143].
The genera Lactobacillus and Bifidobacterium are the most commonly used probiotic bacteria, followed by Streptococcus, Escherichia, Enterococcus, and Bacillus. Some Saccharomyces fungal strains can also be used as probiotics [103] (Table 2).
Table 2.
List of probiotics strains commonly used.
| Lactobacilli | Bifidobacteria | Saccharomyces | Other species |
|---|---|---|---|
| L. acidophilus | B. adolescentis | S. boulardii | Bacillus subtilis |
| L. casei | B. animalis | S. cerevisiae | Enterococcus faecalis |
| L. crispatus | B. bifidum | Escherichia coli | |
| L. fermentum | B. breve | Lactococcus lactis | |
| L. gallinarum | B. infantis | Streptococcus thermophilus | |
| L. gasseri | B. longum | ||
| L. helveticus | |||
| L. johnsonii | |||
| L. lactis | |||
| L. paracasei | |||
| L. plantarum | |||
| L. reuteri | |||
| L. rhamnosus |
For instance, treatment with Lactobacillus rhamnosus GG, a lactic acid bacterium, has been shown to reduce oxidative stress and inflammation in the intestine, modulate the altered microbiota, as well as restore the gut barrier function [144,145,146]; further, it has been reported that the Lactobacillus acidophilus can prevent intestinal inflammation, by reducing the expression of proinflammatory cytokines (IL-6, TNFα, IL-1b, and IL-17) and promoting the production of IL-10, as well as by modulating the GM, favoring the increase of beneficial bacteria [147,148]. Like other Lactobacilli, also the treatment with L. Plantarum has the potential to change the composition of GM, increase SCFAs levels, and decrease the expression of some inflammatory cytokines (such as TNFα, IL1-β, and IL-6), thus preventing metabolic disorders and gut inflammation [149]. In addition to Lactobacilli, bacteria of the genus Bifidobacterium (such as B. bifidum, B. breve, and B. longum) can also be used as probiotics to modify and stabilize the composition of GM, to inhibit the growth of pathogenic bacteria and the production of proinflammatory cytokines, and to strengthen the gastrointestinal barrier [107]. Additionally, it has been reported that the probiotic bacterium Escherichia coli Nissle can modulate the bacterial population of GM and restore the intestinal homeostasis, by producing human β-defensin 2, which is useful as a barrier against the invasion of pathogens (such as Salmonella, Shigella, and Candida) across the intestinal barrier [150,151]. Further, some yeasts, including Saccharomyces cerevisiae and Saccharomyces boulardii, are also employed as probiotics. They have the ability to modify the GM microorganisms and reduce inflammation [107,152]. Interestingly, in addition to these classic probiotics, several studies have shown the beneficial role of other bacteria, known as “next-generation probiotics” (NGP), such as Faecalibacterium prausnitzii and A. muciniphila. For instance, A. muciniphila, a Gram-negative anaerobic bacterium, has been demonstrated to be able to reduce gut inflammation and strengthen the intestinal barrier, favoring the synthesis of antimicrobial substances, the thickening of mucus, and the restoration of tight junctions proteins expression [153,154].
As previously mentioned, the GM plays a dominant role in the pathogenesis of NAFLD [155]. Changes in its composition (for instance, an increase in Gram-negative bacteria belonging to Proteobacteria, Escherichia, and Enterobacteria species) increase intestinal permeability, resulting in the translocation of endotoxins and toxic metabolites into the liver, and leading to the production of inflammatory cytokines by Kupffer cells [156,157,158]. Furthermore, dysbiosis can also alter the metabolism of bile acids and choline, and increase the production of endogenous ethanol in the intestine. All these events cause inflammation and OS, which in turn trigger the onset of the disease and eventually its progression into cirrhosis [54].
To date, no specific drugs have yet been approved to treat NAFLD. The current strategies employed to control the disease and its progression include lifestyle changes (diet modifications, exercise, and gradual weight loss), the use of hypoglycemic and antioxidant agents, as well as drugs commonly used to treat diabetes mellitus (such as metformin and thiazolidinediones) [159]. The employment of probiotics may provide a new therapeutic approach for managing and treating liver diseases, like NAFLD. In fact, as previously discussed, it is well known that they are able to restore the GM to a healthy state, by improving the expression of occludins and blocking the invasion of pathogenic bacteria and endotoxins into the intestine, and to reduce hepatic inflammation, by balancing the expression of pro-and anti-inflammatory cytokines [54,160,161]. Further, they can also favor the production of SCFAs, decrease the amount of hepatic triglycerides, and relieve the intestinal OS, by increasing the levels of the enzymes superoxide dismutase (SOD) and plasma glutathione peroxidase (GSH-PH) and reducing the content of malondialdehyde (MDA) [155,162,163].
To conclude, it is widely reported that the GM is altered in elderly people, and dysbiosis is linked to the onset of NAFLD [136,155]. Thus, since changes in GM can contribute to the irregular synthesis of bile acids, resulting in the excessive accumulation of fats in the liver and development of the disease, the use of probiotics to restore GM composition could be effective to modulate bile acids production and manage NAFLD [55].
7.1. Preclinical Studies of Probiotic Supplementation in NAFLD
Several animal studies have been conducted to evaluate the possible effects of probiotics on NAFLD development and progression (Table 3). It has been reported that Lactobacillus plantarum NCU116 and Lactobacillus plantarum NA136 could be safe probiotics for NAFLD. Notably, L. plantarum NCU116 had beneficial effects in NAFLD model rats, by inhibiting inflammation (decrease TNFα and IL-6 expression) and hepatic oxidative stress (increase SOD, GSH-Px, and catalase activities), and by restoring bacteria flora [164], while Lactobacillus plantarum NA136 could alleviate NAFLD in mice, by increasing nuclear factor erythroid 2-related factor 2 (Nrf2) and AMP-activated protein kinase (AMPK) cascades, resulting in the activation of different antioxidant pathways and regulation of the fatty acid metabolism [165]. Further, it has been shown that Lactobacillus johnsonii BS15 may prevent NAFLD in obese mice, by improving mitochondrial dysfunction and reducing inflammation and gut permeability [166], while treatment with Lactobacillus rhamnosus GG could protect mice and rats from NAFLD, by reducing liver fat accumulation and inflammation (decrease TNFα, IL-1β, and IL-8R mRNA expression) [167], and stimulating sirtuins type 1 (SIRT1)-mediated signaling pathway [168], respectively. In other studies, supplementation with Bifidobacterium longum attenuated liver fat accumulation in NAFLD model rats [169]; treatment with a mixture of probiotics (Bacillus animalis VKB, Bacullus animalis VKL, Lactobacillus casei IMV B-7280) modulated the GM composition and reduced cholesterol level, oxidative stress, and weight in obese mice [170]; and administration of Bifidobacterium infantis, Lactobacillus acidopilus, and Bacillus cereus in rats restored the GM structure, and decreased serum levels of gut-derived bacterial lipopolysaccharide (LPS) and inflammatory cytokines (TNFα and IL-18), and liver toll-like receptor 4 (TLR4)-mRNA [171]. Another study in rats revealed that supplementation with Clostridium butyricum MIYAIRI 588 could improve NAFLD, by decreasing accumulation of lipids droplets [172]. Finally, treatment with VSL#3 probiotics alleviated obesity, hepatic steatosis, and insulin resistance, as well as reduced inflammation, downregulating the activation of TNFα/inhibitor of nuclear factor kappa-B kinase subunit beta (IKK-β) signaling pathway in high-fat diet-fed mice [173]. Moreover, VSL#3 probiotics may reduce alanine aminotransferase (ALT) levels and hepatic total fatty acid in high-fat diet model mice [174].
Table 3.
Effects of probiotics treatment in animals and human experimental studies.
| Probiotic | Model | Diet | Duration | Treatment Effects | Reference |
|---|---|---|---|---|---|
| Lactobacillus rhamnosus GG | Mice | High-fructose diet-induced NAFLD | 8 weeks | 1. Improvement of the accumulation of fat in the liver 2. Reduction of liver inflammation (↓TNFα, ↓IL-8R, ↓IL-1β), as well as steatosis 3. Increase in gut beneficial bacteria 4. Restoration of tight junction proteins, resulting in gut barrier function amelioration |
[167] |
| Lactobacillus rhamnosus GG and Lactobacillus plantarum WCFS1 | Sprague-Dawley rats | High-fat diet-induced NAFLD | 21 weeks | 1. Reduction of gut endotoxemia level, as well as the expression of inflammatory cytokines 2. Amelioration of GM and intestinal barrier function 3. Increase in CYP7A1 and LDL-R, resulting in improvement of lipid metabolism and insulin resistance |
[183] |
| Bifidobacterium infantis , Lactobacillus acidopilus, Bacillus cereus | Rats | High-fat/high-sucrose diet-induced NAFLD | 12 weeks | 1. Downregulation of LPS/TLR4 signaling pathway, resulting in slowing the progression of NAFLD 2. Improvement of GM dysbiosis and the intestinal barrier function 3. Reduction of body weight 4. Decrease in TNFα, and IL-18 expression, as well as ALT, AST, GGT, and ALP activities |
[171] |
| Lactobacillus plantarum ATG-K2 and ATG-K6 | Wistar rats | High-fat and fructose-diet-induced NAFLD | 8 weeks | 1. Modulation of GM 2. Downregulating of de novo lipogenesis-associated genes 3. Reduction of body weight and hepatic lipid accumulation 4. Increasing of antioxidant enzymes (SOD, GPx, CAT), and decreasing of ALT and AST serum levels |
[184] |
| Bifidobacterium animalis subsp. Lactis V9 | Wistar rats | High-fat diet-induced NAFLD | 9 weeks | 1. Decrease in ALT, AST, TLR4, and TLR9 levels, resulting in alleviation of hepatic steatosis and liver damage 2. Reduction of serum glucose level, as well as hepatic triglycerides and free fatty acids accumulation 3. Restoration of hepatic phosphorylated-AMPK and PPAR-α levels, and reduction of SREBP-1c and FAS expression 4. Attenuation of liver inflammation, by inhibiting inflammatory cytokines synthesis (IL-6, IL-1β, TNFα) |
[185] |
| Lactobacillus acidophilus La5 , Bifidobacterium lactis Bb12 | 72 NAFLD patients | 8 weeks | 1. Decreasing of ALT and AST activity 2. Reduction of triglycerides and low-density lipoprotein cholesterol serum levels, as well as total cholesterol |
[186] | |
| Multiprobiotic “Lactocare” (L. casei, L. acidophilus, L. rhamnosus, L. bulgaricus, B. breve, B. longum, Streptococcus thermophilus) | 42 NAFLD patients | 8 weeks | 1. Decrease in TNFα and IL-6 expression, as well as FBS and insulin | [178] | |
| Probiotics mixture (Bifidobacterium, Lactobacillus, and Enterococcus; Bacillus subtilis and Enterococcus) | 200 NAFLD patients | 1 month | 1. Improvement of GM composition, by inhibiting TNFα expression and ameliorating adiponectin level 2. Decrease in ALT and AST serum levels 3. Amelioration of lipid metabolism and fatty liver |
[157] | |
| Multiprobiotic “Symbiter” (Bifidobacterium, Lactobacillus, Lactococcus, Propionibacterium, Acetobacter) | 58 NAFLD patients | 8 weeks | 1. Reduction of liver fat (↓total cholesterol and ↓triglycerides) 2. Decreasing of AST and GGT activity, as well as TNFα and IL-6 expression |
[182] | |
| Lactobacillus paracasei DSM 24733 , Lactobacillus plantarum DSM 24730, Lactobacillus acidophilus DSM 24735 and Lactobacillus delbrueckii subsp. bulgaricus DSM 24734, Bifidobacterium longum DSM 24736, Bifidobacterium infantis DSM 24737, Bifidobacterium breve DSM 24732, and Streptococcus thermophilus DSM 24731 | 30 NAFLD patients | 12 months | 1. Improvement of liver histology 2. Reduction in steatohepatitis 3. Decrease in ALP, AST, and ALT activity, as well as endotoxins, TNFα, IL-1β, and IL-6 levels |
[187] |
Abbreviations: ALP: alkaline phosphatase; ALT: alanine aminotransferase; AMPK: AMP-activated protein kinase; AST: aspartate aminotransferase; CAT: catalase; CYP7A1: cholesterol 7 α-hydroxylase; FAS: lipogenic enzyme fatty acid synthase; FBS: fasting blood sugar; GPx: glutathione peroxidase; GGT: gamma glutamyl transferase; IL-1β: interleukin 1β; IL-6: interleukin 6; IL-8R: interleukin 8 receptor; IL-18: interleukin 18; LDL-R: low-density lipoprotein receptor; LPS/TLR4: lipopolysaccharide/toll-like receptor 4; PPAR-α: peroxisome proliferator-activated receptor α; SOD: superoxide dismutase; SREBP-1c: sterol-regulatory element binding protein-1c; TLR9: toll-like receptor 9; TNFα: tumor necrosis factor α.
In addition to traditional probiotics, the emerging NGP, including, A. muciniphila, F. prausnitzii, Bacteroides spp., and the Roseburia, could represent a potential therapeutic strategy for the treatment of NAFLD [155]. For instance, Munukka (2017). reported the ability of F. prausnitzii probiotic to improve hepatic health, by decreasing fibrosis, aspartate aminotransferase (AST) and ALT levels, and fat content in liver of high-fat fed mice [175].
7.2. Clinical Trials of Probiotic Supplementation in NAFLD
Some human studies have demonstrated the benefits of probiotic supplementation in patients with NAFLD (Table 3). It has been demonstrated that administration of conventional yogurt, fermented by Lactobacillus delbrueckii ssp. bulgaricus and Streptococcus thermophiles, as well as the supplementation of a mixture of six probiotics (L. acidophilus, L. rhamnosus, L. paracasei, Pediococcus pentosaceus, B. lactis, and B. breve) can have beneficial effects on patients with NAFLD, by modifying the GM composition, and reducing inflammation (decrease TNFα expression) and lipid metabolism (decrease total cholesterol and triglycerides) [176,177]. A randomized, double-blind, placebo-controlled clinical trial showed that multistrain probiotic supplementation can decrease insulin, insulin resistance, TNFα, and IL-6 in patients with NAFLD [178]; further, in the same line, treatment with Lactobacillus bulgaricus and Streptococcus thermophilus can decrease ALT and AST activity, and gamma glutamyl transferase (GGT) levels in NAFLD patients [179]. Another randomized, double-blind, placebo-controlled clinical trial reports that administration of VSL#3 decreased triglycerides and high-sensitivity C-reactive protein levels, as well as transaminases and GGT activity [180]. Interestingly, Shavakhi et al. demonstrated that treatment with Metformin plus Protexin (L. acidophilus, L. casei, L. rhamnosus, L. bulgaricus, B. breve, B. longum, Streptococcus thermophilus) decreases ALT and AST activity, better than Metformin alone in patients with NASH [181]. Finally, the use of a cocktail of 14 probiotic strains, belonging to Lactobacillus + Lactococcus, Bifidobacterium, Propionibacterium, and Acetobacter genera, could improve hepatic steatosis, by reducing AST and GGT activity, as well as TNFα and IL-6 levels, in NAFLD patients [182].
8. Other Therapeutic Options
As widely reported, GM modulation represents a valid approach to manage many diseases, including NAFLD. In addition to probiotics, prebiotics, symbiotics, and the so-called fecal microbiota transplant (FMT) represent other methods used to restore dysbiosis [188,189].
Prebiotics are “non-digestible food ingredients that beneficially affect the host’s health, by selectively stimulating the growth and/or activity of beneficial bacteria in the gastrointestinal tract” [190]. Most of them are non-digestible fibers, such as fructo-oligosaccharides (FOS), galacto-oligosaccharides (GOS), lactulose, inulin, and pectin [191]. They can prevent diarrhea, as well as cancer, modulate the metabolism of the intestinal flora, stimulate mineral adsorption, and have positive effects on lipid metabolism and immunomodulatory properties [192]. In addition, prebiotics can modulate the composition of GM, by promoting the growth of beneficial microorganisms and reducing the number of Gram-negative bacteria [193,194,195]. Some evidence showed that prebiotic supplementation can prevent NAFLD development and progression [196,197]. Studies report that prebiotic fructo-oligosaccharides restored normal gastrointestinal microflora and intestinal epithelial barrier function, and decreased steatohepatitis in NASH model mice, while lactulose improved hepatic inflammation and decreased ALT and AST serum level in NASH model rats [198,199]. Moreover, a randomized, double-blind, placebo-controlled clinical trial reported that Chlorella vulgaris can decrease serum glucose level and improve liver function and lipid profile in NAFLD patients [200]; further, in the same line, Javadi (2017) showed that prebiotic inulin reduces AST and ALT levels, compared to placebo. However, they found no significant changes in the grade of fatty liver [201]. Finally, administration of oligofructose decreased ALT, AST, and insulin serum level in patients with NASH [197]. Interestingly, some studies report the effects of prebiotics on the GM in elderly people [202,203,204]. Two randomized, double-blind, placebo-controlled clinical trials show that galacto-oligosaccharides mixture (B-GOS) increased the number of beneficial bacteria, especially Bifidobacteria [202,203], as well as GOS supplementation [204].
Symbiotics are the combination of probiotics and prebiotics, where prebiotics favor the proliferation of healthy probiotics microorganisms, thus creating a beneficial gastrointestinal system, resulting in positive effects to the host’s health [188,192]. Symbiotics should be created by selecting an appropriate combination of probiotics and prebiotics, in order to promote the growth and survival of probiotics in the intestinal tract. Furthermore, the symbiotic formula should be more effective compared to the activity of the individual components [205]. Some studies report the beneficial effects of symbiotic supplementation in biochemical and histological features of NAFLD [206,207,208,209,210,211,212]. Malaguarnera et al. found that the combination of B. longum and FOS, together with lifestyle modification, reduces AST, TNFα, and C-reactive protein (CRP) levels, HOMA index and serum endotoxin, as well as decreases inflammation and steatosis, in 66 NASH patients [206]; moreover, a randomized, double-blind, placebo-controlled clinical trial showed that supplementation of seven probiotic strains (L. casei, L. rhamnosus, S. thermophilus, B. breve, L. acidophilus, B. longum, and L. bulgaricus) and FOS significantly reduced liver enzymes (ALT, AST, and GGT), and inflammatory markers (TNFα, CRP, and total nuclear factor k-B p65) in 52 patients with NAFLD [207]. Another randomized, double-blind, placebo-controlled clinical trial reported that the combination of dietary fiber and L. reuteri reduced fibrosis, hepatic steatosis, and serum levels of inflammatory markers in 50 lean patients with NAFLD [208]. Finally, in a recent clinical trial (the INSYTE study), Scorletti (2020). observed that Bifidobacterium animalis subsp. lactis BB-12 and FOS alter fecal microbiome, but do not reduce liver fat content and markers of liver fibrosis [209]. Symbiotics have the ability to modulate the GM of the elderly [213,214,215]. Two double-blind, placebo-controlled clinical trials report that the mixture of Bifidobacterium bifidum BB-02, Bifidobacterium lactis BL-01, and inulin, as well as mixture of Lactobacillus acidophilus NCFM and lactitol can increase the growth of Bifidobacteria and Lactobacilli [213,215]; in addition, another clinical trial shows that the combination of Bifidobacterium longum and inulin increased the number of Actinobacteria and Firmicutes, and decreased Proteobacteria [214]. Interestingly, Marìa Juàrez-Fernàndez et al. observed the beneficial effect of the symbiotic combination of the NGP A. muciniphila and quercetin on NAFLD, by modulating GM composition and bile acid metabolism [216].
Fecal microbiota transplant (FMT) is the process by which fecal material from healthy donors is inserted into the intestine of patients with an altered GM, in order to restore it to a stable state and thus treat specific diseases related to dysbiosis [217]. At present, FMT has been used successfully in patients with recurrent Clostridum difficile infection, metabolic syndrome, inflammatory bowel syndrome, and obesity [218], and could become an effective therapeutic method for the treatment of NAFLD. It has been demonstrated that restoration of a healthy GM with FMT treatment alleviated steatohepatitis in HFD model mice [219], and restored portal hypertension, insulin resistance, and endothelial dysfunction in NASH model rats [220]. To date, limited human studies have been conducted, and not all have shown beneficial effects of FMT in the treatment of NAFLD. For instance, a double blind, randomized, controlled proof-of-principle study reported that allogenic donor FMT in individuals with hepatic steatosis produced beneficial changes in hepatic gene expression and in metabolites involved in inflammation and lipid metabolism [221]; in addition, another randomized, controlled trial shows that allogenic FMT in patients with NAFLD can reduce small intestinal permeability, but do not improve insulin resistance nor reduce hepatic fat fraction [222].
9. Conclusions
NAFLD is a common liver disease, especially widespread among elderly people with metabolic disorders, which is characterized by excessive fat accumulation in hepatocytes. Several experimental studies conducted both in aged animals, (in which the pathological symptoms of the disease are induced by high-fat and MCD diets) and in adult patients with NAFLD, have highlighted the presence of an altered GM, compared to the one observed in healthy people. In elderly people, the GM is characterized by a particular microbial signature (increase in Gram-negative bacteria and pathobionts, with a consequent release of endotoxins and LPS, and reduction in Gram-positive microorganisms), and this altered GM seems to play a relevant role in promoting the pathogenesis of NAFLD. In fact, the intestinal dysbiosis, together with a high level of OS, determines an increase in the intestinal permeability with a consequent release of ROS, endotoxins, and LPS into the bloodstream. All together, these events lead to an increased susceptibility to develop the disease and favor its progression into NASH. Therefore, as several experimental studies and clinical trials highlight, the restoration of the altered GM to a healthy state could be a new beneficial weapon to manage NAFLD. Probiotics supplementation, alone or in combination with NAFLD traditional treatments, could then represent a new therapeutic approach capable of reinstating a balanced intestinal flora, even if their synergic action is not yet well known. Indeed, although probiotics have been used for decades to prevent or treat some disorders, to date, their efficacy in counteracting or alleviating NAFLD has not yet been fully explored. In fact, although promising, both preclinical researches and randomized controlled trials are still few to demonstrate therapeutic efficacy in NAFLD management. Moreover, more studies are required, on one side, to better clarify the precise role of the altered GM in the pathogenesis of this hepatic disease, and on the other, to find the most effective probiotic strains that can be used, the dosage to be administered, and the duration of the treatment.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
We acknowledge the financial contribution of FRG2021 from the University of Pavia.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.de Vos W.M., Tilg H., Van Hul M., Cani P.D. Gut Microbiome and Health: Mechanistic Insights. Gut. 2022;71:1020–1032. doi: 10.1136/gutjnl-2021-326789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu H.-J., Wu E. The Role of Gut Microbiota in Immune Homeostasis and Autoimmunity. Gut Microbes. 2012;3:4–14. doi: 10.4161/gmic.19320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Y., Zhou J., Wang L. Role and Mechanism of Gut Microbiota in Human Disease. Front. Cell. Infect. Microbiol. 2021;11:625913. doi: 10.3389/fcimb.2021.625913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacNee W., Rabinovich R.A., Choudhury G. Ageing and the Border between Health and Disease. Eur. Respir. J. 2014;44:1332–1352. doi: 10.1183/09031936.00134014. [DOI] [PubMed] [Google Scholar]
- 5.Stahl E.C., Haschak M.J., Popovic B., Brown B.N. Macrophages in the Aging Liver and Age-Related Liver Disease. Front. Immunol. 2018;9:2795. doi: 10.3389/fimmu.2018.02795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papatheodoridi A., Chrysavgis L., Koutsilieris M., Chatzigeorgiou A. The Role of Senescence in the Development of Nonalcoholic Fatty Liver Disease and Progression to Nonalcoholic Steatohepatitis. Hepatology. 2020;71:363–374. doi: 10.1002/hep.30834. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez A., Huerta-Salgado C., Orozco-Aguilar J., Aguirre F., Tacchi F., Simon F., Cabello-Verrugio C. Role of Oxidative Stress in Hepatic and Extrahepatic Dysfunctions during Nonalcoholic Fatty Liver Disease (NAFLD) Oxidative Med. Cell. Longev. 2020;2020:1–16. doi: 10.1155/2020/1617805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang X., Zheng J., Zhang S., Wang B., Wu C., Guo X. Advances in the Involvement of Gut Microbiota in Pathophysiology of NAFLD. Front. Med. 2020;7:361. doi: 10.3389/fmed.2020.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hrncir T., Hrncirova L., Kverka M., Hromadka R., Machova V., Trckova E., Kostovcikova K., Kralickova P., Krejsek J., Tlaskalova-Hogenova H. Gut Microbiota and NAFLD: Pathogenetic Mechanisms, Microbiota Signatures, and Therapeutic Interventions. Microorganisms. 2021;9:957. doi: 10.3390/microorganisms9050957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arab J.P., Arrese M., Trauner M. Recent Insights into the Pathogenesis of Nonalcoholic Fatty Liver Disease. Annu. Rev. Pathol. Mech. Dis. 2018;13:321–350. doi: 10.1146/annurev-pathol-020117-043617. [DOI] [PubMed] [Google Scholar]
- 11.Buzzetti E., Pinzani M., Tsochatzis E.A. The Multiple-Hit Pathogenesis of Non-Alcoholic Fatty Liver Disease (NAFLD) Metabolism. 2016;65:1038–1048. doi: 10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Lin W.-Y., Lin J.-H., Kuo Y.-W., Chiang P.-F.R., Ho H.-H. Probiotics and Their Metabolites Reduce Oxidative Stress in Middle-Aged Mice. Curr. Microbiol. 2022;79:104. doi: 10.1007/s00284-022-02783-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Pasqua L.G., Cagna M., Berardo C., Vairetti M., Ferrigno A. Detailed Molecular Mechanisms Involved in Drug-Induced Non-Alcoholic Fatty Liver Disease and Non-Alcoholic Steatohepatitis: An Update. Biomedicines. 2022;10:194. doi: 10.3390/biomedicines10010194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berardo C., Di Pasqua L.G., Cagna M., Richelmi P., Vairetti M., Ferrigno A. Nonalcoholic Fatty Liver Disease and Non-Alcoholic Steatohepatitis: Current Issues and Future Perspectives in Preclinical and Clinical Research. Int. J. Mol. Sci. 2020;21:9646. doi: 10.3390/ijms21249646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Younossi Z.M., Rinella M.E., Sanyal A.J., Harrison S.A., Brunt E.M., Goodman Z., Cohen D.E., Loomba R. From NAFLD to MAFLD: Implications of a Premature Change in Terminology. Hepatology. 2021;73:1194–1198. doi: 10.1002/hep.31420. [DOI] [PubMed] [Google Scholar]
- 16.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global Epidemiology of Nonalcoholic Fatty Liver Disease-Meta-Analytic Assessment of Prevalence, Incidence, and Outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 17.Jinjuvadia R., Antaki F., Lohia P., Liangpunsakul S. The Association between Nonalcoholic Fatty Liver Disease and Metabolic Abnormalities in the United States Population. J. Clin. Gastroenterol. 2017;51:160–166. doi: 10.1097/MCG.0000000000000666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Younossi Z.M., Golabi P., de Avila L., Paik J.M., Srishord M., Fukui N., Qiu Y., Burns L., Afendy A., Nader F. The Global Epidemiology of NAFLD and NASH in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. J. Hepatol. 2019;71:793–801. doi: 10.1016/j.jhep.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 19.Ferrigno A., Berardo C., Di Pasqua L.G., Cagna M., Siciliano V., Richelmi P., Vairetti M. The Selective Blockade of Metabotropic Glutamate Receptor-5 Attenuates Fat Accumulation in an In Vitro Model of Benign Steatosis. Eur. J. Histochem. 2020;64:3175. doi: 10.4081/ejh.2020.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peverill W., Powell L.W., Skoien R. Evolving Concepts in the Pathogenesis of NASH: Beyond Steatosis and Inflammation. Int. J. Mol. Sci. 2014;15:8591–8638. doi: 10.3390/ijms15058591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Day C.P., Saksena S. Non-Alcoholic Steatohepatitis: Definitions and Pathogenesis. J. Gastroenterol. Hepatol. 2002;17:S377–S384. doi: 10.1046/j.1440-1746.17.s3.31.x. [DOI] [PubMed] [Google Scholar]
- 22.Hebbard L., George J. Animal Models of Nonalcoholic Fatty Liver Disease. Nat. Rev. Gastroenterol. Hepatol. 2011;8:35–44. doi: 10.1038/nrgastro.2010.191. [DOI] [PubMed] [Google Scholar]
- 23.Guilherme A., Virbasius J.V., Puri V., Czech M.P. Adipocyte Dysfunctions Linking Obesity to Insulin Resistance and Type 2 Diabetes. Nat. Rev. Mol. Cell Biol. 2008;9:367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gong Z., Tas E., Yakar S., Muzumdar R. Hepatic Lipid Metabolism and Non-Alcoholic Fatty Liver Disease in Aging. Mol. Cell. Endocrinol. 2017;455:115–130. doi: 10.1016/j.mce.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 25.Barzilai N., Huffman D.M., Muzumdar R.H., Bartke A. The Critical Role of Metabolic Pathways in Aging. Diabetes. 2012;61:1315–1322. doi: 10.2337/db11-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Postic C., Girard J. Contribution of de Novo Fatty Acid Synthesis to Hepatic Steatosis and Insulin Resistance: Lessons from Genetically Engineered Mice. J. Clin. Investig. 2008;118:829–838. doi: 10.1172/JCI34275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu X., So J.-S., Park J.-G., Lee A.-H. Transcriptional Control of Hepatic Lipid Metabolism by SREBP and ChREBP. Semin. Liver Dis. 2013;33:301–311. doi: 10.1055/s-0033-1358523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gruzdeva O., Borodkina D., Uchasova E., Dyleva Y., Barbarash O. Leptin Resistance: Underlying Mechanisms and Diagnosis. Diabetes Metab. Syndr. Obes. 2019;12:191–198. doi: 10.2147/DMSO.S182406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendoza-Herrera K., Florio A.A., Moore M., Marrero A., Tamez M., Bhupathiraju S.N., Mattei J. The Leptin System and Diet: A Mini Review of the Current Evidence. Front. Endocrinol. 2021;12:749050. doi: 10.3389/fendo.2021.749050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Margetic S., Gazzola C., Pegg G., Hill R. Leptin: A Review of Its Peripheral Actions and Interactions. Int. J. Obes. 2002;26:1407–1433. doi: 10.1038/sj.ijo.0802142. [DOI] [PubMed] [Google Scholar]
- 31.Muoio D.M., Lynis Dohm G. Peripheral Metabolic Actions of Leptin. Best Pract. Res. Clin. Endocrinol. Metab. 2002;16:653–666. doi: 10.1053/beem.2002.0223. [DOI] [PubMed] [Google Scholar]
- 32.Cusi K. Role of Insulin Resistance and Lipotoxicity in Non-Alcoholic Steatohepatitis. Clin. Liver Dis. 2009;13:545–563. doi: 10.1016/j.cld.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 33.Yilmaz Y. Review Article: Is Non-Alcoholic Fatty Liver Disease a Spectrum, or Are Steatosis and Non-Alcoholic Steatohepatitis Distinct Conditions? Aliment. Pharmacol. Ther. 2012;36:815–823. doi: 10.1111/apt.12046. [DOI] [PubMed] [Google Scholar]
- 34.Li H.-Y., Peng Z.-G. Targeting Lipophagy as a Potential Therapeutic Strategy for Nonalcoholic Fatty Liver Disease. Biochem. Pharmacol. 2022;197:114933. doi: 10.1016/j.bcp.2022.114933. [DOI] [PubMed] [Google Scholar]
- 35.Carotti S., Aquilano K., Zalfa F., Ruggiero S., Valentini F., Zingariello M., Francesconi M., Perrone G., Alletto F., Antonelli-Incalzi R., et al. Lipophagy Impairment Is Associated with Disease Progression in NAFLD. Front. Physiol. 2020;11:850. doi: 10.3389/fphys.2020.00850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grefhorst A., van de Peppel I.P., Larsen L.E., Jonker J.W., Holleboom A.G. The Role of Lipophagy in the Development and Treatment of Non-Alcoholic Fatty Liver Disease. Front. Endocrinol. 2021;11:601627. doi: 10.3389/fendo.2020.601627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cӑtoi A.F., Corina A., Katsiki N., Vodnar D.C., Andreicuț A.D., Stoian A.P., Rizzo M., Pérez-Martínez P. Gut Microbiota and Aging-A Focus on Centenarians. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2020;1866:165765. doi: 10.1016/j.bbadis.2020.165765. [DOI] [PubMed] [Google Scholar]
- 38.Lloyd-Price J., Abu-Ali G., Huttenhower C. The Healthy Human Microbiome. Genome Med. 2016;8:51. doi: 10.1186/s13073-016-0307-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Juárez-Fernández M., Porras D., García-Mediavilla M.V., Román-Sagüillo S., González-Gallego J., Nistal E., Sánchez-Campos S. Aging, Gut Microbiota and Metabolic Diseases: Management through Physical Exercise and Nutritional Interventions. Nutrients. 2020;13:16. doi: 10.3390/nu13010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marciano F., Vajro P. Gastrointestinal Tissue. Elsevier; Amsterdam, The Netherlands: 2017. Oxidative Stress and Gut Microbiota; pp. 113–123. [Google Scholar]
- 41.Collado M.C., Rautava S., Aakko J., Isolauri E., Salminen S. Human Gut Colonisation May Be Initiated in Utero by Distinct Microbial Communities in the Placenta and Amniotic Fluid. Sci. Rep. 2016;6:23129. doi: 10.1038/srep23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohajeri M.H., Brummer R.J.M., Rastall R.A., Weersma R.K., Harmsen H.J.M., Faas M., Eggersdorfer M. The Role of the Microbiome for Human Health: From Basic Science to Clinical Applications. Eur J. Nutr. 2018;57:1–14. doi: 10.1007/s00394-018-1703-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Claesson M.J., Cusack S., O’Sullivan O., Greene-Diniz R., de Weerd H., Flannery E., Marchesi J.R., Falush D., Dinan T., Fitzgerald G., et al. Composition, Variability, and Temporal Stability of the Intestinal Microbiota of the Elderly. Proc. Natl. Acad. Sci. USA. 2011;108:4586–4591. doi: 10.1073/pnas.1000097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lozupone C.A., Stombaugh J.I., Gordon J.I., Jansson J.K., Knight R. Diversity, Stability and Resilience of the Human Gut Microbiota. Nature. 2012;489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santoro A., Ostan R., Candela M., Biagi E., Brigidi P., Capri M., Franceschi C. Gut Microbiota Changes in the Extreme Decades of Human Life: A Focus on Centenarians. Cell. Mol. Life Sci. 2018;75:129–148. doi: 10.1007/s00018-017-2674-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dam B., Misra A., Banerjee S. Role of Gut Microbiota in Combating Oxidative Stress. In: Chakraborti S., Chakraborti T., Chattopadhyay D., Shaha C., editors. Oxidative Stress in Microbial Diseases. Springer; Singapore: 2019. pp. 43–82. [Google Scholar]
- 47.Jasirwan C.O.M., Lesmana C.R.A., Hasan I., Sulaiman A.S., Gani R.A. The Role of Gut Microbiota in Non-Alcoholic Fatty Liver Disease: Pathways of Mechanisms. Biosci. Microbiota Food Health. 2019;38:81–88. doi: 10.12938/bmfh.18-032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones R.M., Mercante J.W., Neish A.S. Reactive Oxygen Production Induced by the Gut Microbiota: Pharmacotherapeutic Implications. CMC. 2012;19:1519–1529. doi: 10.2174/092986712799828283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Knights D., Parfrey L.W., Zaneveld J., Lozupone C., Knight R. Human-Associated Microbial Signatures: Examining Their Predictive Value. Cell Host Microbe. 2011;10:292–296. doi: 10.1016/j.chom.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baumann A., Hernández-Arriaga A., Brandt A., Sánchez V., Nier A., Jung F., Kehm R., Höhn A., Grune T., Frahm C., et al. Microbiota Profiling in Aging-Associated Inflammation and Liver Degeneration. Int. J. Med. Microbiol. 2021;311:151500. doi: 10.1016/j.ijmm.2021.151500. [DOI] [PubMed] [Google Scholar]
- 51.Sharma R. Emerging Interrelationship Between the Gut Microbiome and Cellular Senescence in the Context of Aging and Disease: Perspectives and Therapeutic Opportunities. Probiotics Antimicro. Prot. 2022 doi: 10.1007/s12602-021-09903-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.García-Peña C., Álvarez-Cisneros T., Quiroz-Baez R., Friedland R.P. Microbiota and Aging. A Review and Commentary. Arch. Med. Res. 2017;48:681–689. doi: 10.1016/j.arcmed.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 53.Kim S., Jazwinski S.M. The Gut Microbiota and Healthy Aging: A Mini-Review. Gerontology. 2018;64:513–520. doi: 10.1159/000490615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khan A., Ding Z., Ishaq M., Bacha A.S., Khan I., Hanif A., Li W., Guo X. Understanding the Effects of Gut Microbiota Dysbiosis on Nonalcoholic Fatty Liver Disease and the Possible Probiotics Role: Recent Updates. Int. J. Biol. Sci. 2021;17:818–833. doi: 10.7150/ijbs.56214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sivamaruthi B.S., Fern L.A., Rashidah Pg Hj Ismail D.S.N., Chaiyasut C. The Influence of Probiotics on Bile Acids in Diseases and Aging. Biomed. Pharmacother. 2020;128:110310. doi: 10.1016/j.biopha.2020.110310. [DOI] [PubMed] [Google Scholar]
- 56.Salazar N., Arboleya S., Valdés L., Stanton C., Ross P., Ruiz L., Gueimonde M., de los Reyes-Gavilán C.G. The Human Intestinal Microbiome at Extreme Ages of Life. Dietary Intervention as a Way to Counteract Alterations. Front. Genet. 2014;5 doi: 10.3389/fgene.2014.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Acharya C., Bajaj J.S. Chronic Liver Diseases and the Microbiome—Translating Our Knowledge of Gut Microbiota to Management of Chronic Liver Disease. Gastroenterology. 2021;160:556–572. doi: 10.1053/j.gastro.2020.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alqahtani S.A., Schattenberg J.M. NAFLD in the Elderly. CIA. 2021;16:1633–1649. doi: 10.2147/CIA.S295524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oudshoorn C., van der Cammen T.J.M., McMurdo M.E.T., van Leeuwen J.P.T.M., Colin E.M. Ageing and Vitamin D Deficiency: Effects on Calcium Homeostasis and Considerations for Vitamin D Supplementation. Br. J. Nutr. 2009;101:1597–1606. doi: 10.1017/S0007114509338842. [DOI] [PubMed] [Google Scholar]
- 60.Riaz Rajoka M.S., Thirumdas R., Mehwish H.M., Umair M., Khurshid M., Hayat H.F., Phimolsiripol Y., Pallarés N., Martí-Quijal F.J., Barba F.J. Role of Food Antioxidants in Modulating Gut Microbial Communities: Novel Understandings in Intestinal Oxidative Stress Damage and Their Impact on Host Health. Antioxidants. 2021;10:1563. doi: 10.3390/antiox10101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maier L., Pruteanu M., Kuhn M., Zeller G., Telzerow A., Anderson E.E., Brochado A.R., Fernandez K.C., Dose H., Mori H., et al. Extensive Impact of Non-Antibiotic Drugs on Human Gut Bacteria. Nature. 2018;555:623–628. doi: 10.1038/nature25979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pascale A., Marchesi N., Govoni S., Barbieri A. Targeting the Microbiota in Pharmacology of Psychiatric Disorders. Pharmacol. Res. 2020;157:104856. doi: 10.1016/j.phrs.2020.104856. [DOI] [PubMed] [Google Scholar]
- 63.Santos A.L., Sinha S., Lindner A.B. The Good, the Bad, and the Ugly of ROS: New Insights on Aging and Aging-Related Diseases from Eukaryotic and Prokaryotic Model Organisms. Oxidative Med. Cell. Longev. 2018;2018:1–23. doi: 10.1155/2018/1941285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tan B.L., Norhaizan M.E., Liew W.-P.-P., Sulaiman Rahman H. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018;9:1162. doi: 10.3389/fphar.2018.01162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Conti V., Izzo V., Corbi G., Russomanno G., Manzo V., De Lise F., Di Donato A., Filippelli A. Antioxidant Supplementation in the Treatment of Aging-Associated Diseases. Front. Pharmacol. 2016;7 doi: 10.3389/fphar.2016.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Delli Bovi A.P., Marciano F., Mandato C., Siano M.A., Savoia M., Vajro P. Oxidative Stress in Non-Alcoholic Fatty Liver Disease. An Updated Mini Review. Front. Med. 2021;8:595371. doi: 10.3389/fmed.2021.595371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Houser M.C., Tansey M.G. The Gut-Brain Axis: Is Intestinal Inflammation a Silent Driver of Parkinson’s Disease Pathogenesis? npj Parkinson’s Dis. 2017;3:3. doi: 10.1038/s41531-016-0002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yardeni T., Tanes C.E., Bittinger K., Mattei L.M., Schaefer P.M., Singh L.N., Wu G.D., Murdock D.G., Wallace D.C. Host Mitochondria Influence Gut Microbiome Diversity: A Role for ROS. Sci. Signal. 2019;12:eaaw3159. doi: 10.1126/scisignal.aaw3159. [DOI] [PubMed] [Google Scholar]
- 69.Derrien M., Veiga P. Rethinking Diet to Aid Human–Microbe Symbiosis. Trends Microbiol. 2017;25:100–112. doi: 10.1016/j.tim.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 70.Peng C., Stewart A.G., Woodman O.L., Ritchie R.H., Qin C.X. Non-Alcoholic Steatohepatitis: A Review of Its Mechanism, Models and Medical Treatments. Front. Pharmacol. 2020;11:603926. doi: 10.3389/fphar.2020.603926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gupta H., Min B.-H., Ganesan R., Gebru Y.A., Sharma S.P., Park E., Won S.-M., Jeong J.-J., Lee S.-B., Cha M.-G., et al. Gut Microbiome in Non-Alcoholic Fatty Liver Disease: From Mechanisms to Therapeutic Role. Biomedicines. 2022;10:550. doi: 10.3390/biomedicines10030550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aron-Wisnewsky J., Vigliotti C., Witjes J., Le P., Holleboom A.G., Verheij J., Nieuwdorp M., Clément K. Gut Microbiota and Human NAFLD: Disentangling Microbial Signatures from Metabolic Disorders. Nat. Rev. Gastroenterol. Hepatol. 2020;17:279–297. doi: 10.1038/s41575-020-0269-9. [DOI] [PubMed] [Google Scholar]
- 73.Bäckhed F., Ding H., Wang T., Hooper L.V., Gou Y.K., Nagy A., Semenkovich C.F., Gordon J.I. The Gut Microbiota as an Environmental Factor That Regulates Fat Storage. Proc. Natl. Acad. Sci. USA. 2004;101:15718. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bäckhed F., Manchester J.K., Semenkovich C.F., Gordon J.I. From the Cover: Mechanisms Underlying the Resistance to Diet-Induced Obesity in Germ-Free Mice. Proc. Natl. Acad. Sci. USA. 2007;104:979. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kaden-Volynets V., Basic M., Neumann U., Pretz D., Rings A., Bleich A., Bischoff S.C. Lack of Liver Steatosis in Germ-Free Mice Following Hypercaloric Diets. Eur. J. Nutr. 2019;58:1933–1945. doi: 10.1007/s00394-018-1748-4. [DOI] [PubMed] [Google Scholar]
- 76.Sharpton S.R., Schnabl B., Knight R., Loomba R. Current Concepts, Opportunities, and Challenges of Gut Microbiome-Based Personalized Medicine in Nonalcoholic Fatty Liver Disease. Cell Metab. 2021;33:21–32. doi: 10.1016/j.cmet.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Le Roy T., Llopis M., Lepage P., Bruneau A., Rabot S., Bevilacqua C., Martin P., Philippe C., Walker F., Bado A., et al. Intestinal Microbiota Determines Development of Non-Alcoholic Fatty Liver Disease in Mice. Gut. 2013;62:1787–1794. doi: 10.1136/gutjnl-2012-303816. [DOI] [PubMed] [Google Scholar]
- 78.Chiu C.C., Ching Y.H., Li Y.P., Liu J.Y., Huang Y.T., Huang Y.W., Yang S.S., Huang W.C., Chuang H.L. Nonalcoholic Fatty Liver Disease Is Exacerbated in High-Fat Diet-Fed Gnotobiotic Mice by Colonization with the Gut Microbiota from Patients with Nonalcoholic Steatohepatitis. Nutrients. 2017;9:1220. doi: 10.3390/nu9111220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Porras D., Nistal E., Martínez-Flórez S., Olcoz J.L., Jover R., Jorquera F., González-Gallego J., García-Mediavilla M.V., Sánchez-Campos S. Functional Interactions between Gut Microbiota Transplantation, Quercetin, and High-Fat Diet Determine Non-Alcoholic Fatty Liver Disease Development in Germ-Free Mice. Mol. Nutr. Food Res. 2019;63 doi: 10.1002/mnfr.201800930. [DOI] [PubMed] [Google Scholar]
- 80.Schneider K.M., Bieghs V., Heymann F., Hu W., Dreymueller D., Liao L., Frissen M., Ludwig A., Gassler N., Pabst O., et al. CX3CR1 Is a Gatekeeper for Intestinal Barrier Integrity in Mice: Limiting Steatohepatitis by Maintaining Intestinal Homeostasis. Hepatology. 2015 doi: 10.1002/hep.27982. [DOI] [PubMed] [Google Scholar]
- 81.Palladini G., Di Pasqua L.G., Berardo C., Siciliano V., Richelmi P., Perlini S., Ferrigno A., Vairetti M. Animal Models of Steatosis (NAFLD) and Steatohepatitis (NASH) Exhibit Hepatic Lobe-Specific Gelatinases Activity and Oxidative Stress. Can. J. Gastroenterol. Hepatol. 2019;2019 doi: 10.1155/2019/5413461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ye J.Z., Li Y.T., Wu W.R., Shi D., Fang D.Q., Yang L.Y., Bian X.Y., Wu J.J., Wang Q., Jiang X.W., et al. Dynamic Alterations in the Gut Microbiota and Metabolome during the Development of Methionine-Choline-Deficient Diet-Induced Nonalcoholic Steatohepatitis. World J. Gastroenterol. 2018 doi: 10.3748/wjg.v24.i23.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schneider K.M., Mohs A., Kilic K., Candels L.S., Elfers C., Bennek E., Ben Schneider L., Heymann F., Gassler N., Penders J., et al. Intestinal Microbiota Protects against MCD Diet-Induced Steatohepatitis. Int. J. Mol. Sci. 2019 doi: 10.3390/ijms20020308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Velayudham A., Dolganiuc A., Ellis M., Petrasek J., Kodys K., Mandrekar P., Szabo G. VSL#3 Probiotic Treatment Attenuates Fibrosis without Changes in Steatohepatitis in a Diet-Induced Nonalcoholic Steatohepatitis Model in Mice. Hepatology. 2009 doi: 10.1002/hep.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tilg H., Adolph T.E., Moschen A.R. Multiple Parallel Hits Hypothesis in Nonalcoholic Fatty Liver Disease: Revisited After a Decade. Hepatology. 2021;73:833–842. doi: 10.1002/hep.31518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Spruss A., Kanuri G., Wagnerberger S., Haub S., Bischoff S.C., Bergheim I. Toll-like Receptor 4 Is Involved in the Development of Fructose-Induced Hepatic Steatosis in Mice. Hepatology. 2009;50:1094–1104. doi: 10.1002/hep.23122. [DOI] [PubMed] [Google Scholar]
- 87.Krenkel O., Puengel T., Govaere O., Abdallah A.T., Mossanen J.C., Kohlhepp M., Liepelt A., Lefebvre E., Luedde T., Hellerbrand C., et al. Therapeutic Inhibition of Inflammatory Monocyte Recruitment Reduces Steatohepatitis and Liver Fibrosis. Hepatology. 2018 doi: 10.1002/hep.29544. [DOI] [PubMed] [Google Scholar]
- 88.Schnabl B. Linking Intestinal Homeostasis and Liver Disease. Curr. Opin. Gastroenterol. 2013;29:264–270. doi: 10.1097/MOG.0b013e32835ff948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Henao-Mejia J., Elinav E., Jin C., Hao L., Mehal W.Z., Strowig T., Thaiss C.A., Kau A.L., Eisenbarth S.C., Jurczak M.J., et al. Inflammasome-Mediated Dysbiosis Regulates Progression of NAFLD and Obesity. Nature. 2012 doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gil-Gómez A., Brescia P., Rescigno M., Romero-Gómez M. Gut-Liver Axis in Nonalcoholic Fatty Liver Disease: The Impact of the Metagenome, End Products, and the Epithelial and Vascular Barriers. Semin. Liver Dis. 2021 doi: 10.1055/s-0041-1723752. [DOI] [PubMed] [Google Scholar]
- 91.Velázquez K.T., Enos R.T., Bader J.E., Sougiannis A.T., Carson M.S., Chatzistamou I., Carson J.A., Nagarkatti P.S., Nagarkatti M., Murphy E.A. Prolonged High-Fat-Diet Feeding Promotes Non-Alcoholic Fatty Liver Disease and Alters Gut Microbiota in Mice. World J. Hepatol. 2019;11:619–637. doi: 10.4254/wjh.v11.i8.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gómez-Zorita S., Aguirre L., Milton-Laskibar I., Fernández-Quintela A., Trepiana J., Kajarabille N., Mosqueda-Solís A., González M., Portillo M.P. Relationship between Changes in Microbiota and Liver Steatosis Induced by High-Fat Feeding—A Review of Rodent Models. Nutrients. 2019;11:2156. doi: 10.3390/nu11092156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R., Gordon J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 94.Zhu L., Baker S.S., Gill C., Liu W., Alkhouri R., Baker R.D., Gill S.R. Characterization of Gut Microbiomes in Nonalcoholic Steatohepatitis (NASH) Patients: A Connection between Endogenous Alcohol and NASH. Hepatology. 2013;57:601–609. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]
- 95.Shen F., Zheng R.D., Sun X.Q., Ding W.J., Wang X.Y., Fan J.G. Gut Microbiota Dysbiosis in Patients with Non-Alcoholic Fatty Liver Disease. Hepatobiliary Pancreat. Dis. Int. HBPD INT. 2017;16:375–381. doi: 10.1016/S1499-3872(17)60019-5. [DOI] [PubMed] [Google Scholar]
- 96.Alferink L.J.M., Radjabzadeh D., Erler N.S., Vojinovic D., Medina-Gomez C., Uitterlinden A.G., de Knegt R.J., Amin N., Ikram M.A., Janssen H.L.A., et al. Microbiomics, Metabolomics, Predicted Metagenomics, and Hepatic Steatosis in a Population-Based Study of 1,355 Adults. Hepatology. 2021;73:968–982. doi: 10.1002/hep.31417. [DOI] [PubMed] [Google Scholar]
- 97.Del Chierico F., Nobili V., Vernocchi P., Russo A., De Stefanis C., Gnani D., Furlanello C., Zandonà A., Paci P., Capuani G., et al. Gut Microbiota Profiling of Pediatric Nonalcoholic Fatty Liver Disease and Obese Patients Unveiled by an Integrated Meta-Omics-Based Approach. Hepatology. 2017;65:451–464. doi: 10.1002/hep.28572. [DOI] [PubMed] [Google Scholar]
- 98.Loomba R., Seguritan V., Li W., Long T., Klitgord N., Bhatt A., Dulai P.S., Caussy C., Bettencourt R., Highlander S.K., et al. Gut Microbiome-Based Metagenomic Signature for Non-Invasive Detection of Advanced Fibrosis in Human Nonalcoholic Fatty Liver Disease. Cell Metab. 2017;25:1054–1062.e5. doi: 10.1016/j.cmet.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang B., Jiang X., Cao M., Ge J., Bao Q., Tang L., Chen Y., Li L. Altered Fecal Microbiota Correlates with Liver Biochemistry in Nonobese Patients with Non-Alcoholic Fatty Liver Disease. Sci. Rep. 2016;6:1–11. doi: 10.1038/srep32002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Raman M., Ahmed I., Gillevet P.M., Probert C.S., Ratcliffe N.M., Smith S., Greenwood R., Sikaroodi M., Lam V., Crotty P., et al. Fecal Microbiome and Volatile Organic Compound Metabolome in Obese Humans with Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2013;11:868–875.e3. doi: 10.1016/j.cgh.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 101.Boursier J., Mueller O., Barret M., Machado M., Fizanne L., Araujo-Perez F., Guy C.D., Seed P.C., Rawls J.F., David L.A., et al. The Severity of Nonalcoholic Fatty Liver Disease Is Associated with Gut Dysbiosis and Shift in the Metabolic Function of the Gut Microbiota. Hepatology. 2016;63:764–775. doi: 10.1002/hep.28356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Morelli L., Capurso L. FAO/WHO Guidelines on Probiotics: 10 Years Later. J. Clin. Gastroenterol. 2012;46:S1–S2. doi: 10.1097/MCG.0b013e318269fdd5. [DOI] [PubMed] [Google Scholar]
- 103.Gupta V., Garg R. PROBIOTICS. Indian J. Med. Microbiol. 2009;27:202–209. doi: 10.4103/0255-0857.53201. [DOI] [PubMed] [Google Scholar]
- 104.Kumar H., Salminen S. Encyclopedia of Food and Health. Elsevier; Amsterdam, The Netherlands: 2016. Probiotics; pp. 510–515. [Google Scholar]
- 105.Aeron G., Morya S. Probiotics as Therapeutics. JARB. 2017;2:1–6. doi: 10.15226/2475-4714/2/3/00127. [DOI] [Google Scholar]
- 106.Thomas C.M., Versalovic J. Probiotics-Host Communication: Modulation of Signaling Pathways in the Intestine. Gut Microbes. 2010;1:148–163. doi: 10.4161/gmic.1.3.11712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Azad M.A.K., Sarker M., Li T., Yin J. Probiotic Species in the Modulation of Gut Microbiota: An Overview. BioMed Res. Int. 2018;2018:1–8. doi: 10.1155/2018/9478630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Duncan S.H., Flint H.J. Probiotics and Prebiotics and Health in Ageing Populations. Maturitas. 2013;75:44–50. doi: 10.1016/j.maturitas.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 109.Reid G., Jass J., Sebulsky M.T., McCormick J.K. Potential Uses of Probiotics in Clinical Practice. Clin. Microbiol. Rev. 2003;16:658–672. doi: 10.1128/CMR.16.4.658-672.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sanklecha M., Verma L., Pai U., Mishra S., Maqsood S., Birla A. Lactobacillus Rhamnosus GG Evaluation in Acute Diarrhea (LEAD): An Observational Study. Cureus. 2022 doi: 10.7759/cureus.24594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ishida Y., Nakamura F., Kanzato H., Sawada D., Hirata H., Nishimura A., Kajimoto O., Fujiwara S. Clinical Effects of Lactobacillus Acidophilus Strain L-92 on Perennial Allergic Rhinitis: A Double-Blind, Placebo-Controlled Study. J. Dairy Sci. 2005;88:527–533. doi: 10.3168/jds.S0022-0302(05)72714-4. [DOI] [PubMed] [Google Scholar]
- 112.Surawicz C.M., Elmer G.W., Speelman P., McFarland L.V., Chinn J., Van Belle G. Prevention of Antibiotic-Associated Diarrhea by Saccharomyces Boulardii: A Prospective Study. Gastroenterology. 1989;96:981–988. doi: 10.1016/0016-5085(89)91613-2. [DOI] [PubMed] [Google Scholar]
- 113.Zhang B., An J., Shimada T., Liu S., Maeyama K. Oral Administration of Enterococcus Faecalis FK-23 Suppresses Th17 Cell Development and Attenuates Allergic Airway Responses in Mice. Int. J. Mol. Med. 2012;30:248–254. doi: 10.3892/ijmm.2012.1010. [DOI] [PubMed] [Google Scholar]
- 114.Wang I.-J., Wang J.-Y. Children with Atopic Dermatitis Show Clinical Improvement after Lactobacillus Exposure. Clin. Exp. Allergy. 2015;45:779–787. doi: 10.1111/cea.12489. [DOI] [PubMed] [Google Scholar]
- 115.Kim J.Y., Kwon J.H., Ahn S.H., Lee S.I., Han Y.S., Choi Y.O., Lee S.Y., Ahn K.M., Ji G.E. Effect of Probiotic Mix (Bifidobacterium Bifidum, Bifidobacterium Lactis, Lactobacillus Acidophilus) in the Primary Prevention of Eczema: A Double-Blind, Randomized, Placebo-Controlled Trial. Pediatric Allergy Immunol. 2010;21:e386–e393. doi: 10.1111/j.1399-3038.2009.00958.x. [DOI] [PubMed] [Google Scholar]
- 116.Gaziano R., Sabbatini S., Roselletti E., Perito S., Monari C. Saccharomyces Cerevisiae-Based Probiotics as Novel Antimicrobial Agents to Prevent and Treat Vaginal Infections. Front. Microbiol. 2020;11:718. doi: 10.3389/fmicb.2020.00718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Homayouni A., Bastani P., Ziyadi S., Mohammad-Alizadeh-Charandabi S., Ghalibaf M., Mortazavian A.M., Mehrabany E.V. Effects of Probiotics on the Recurrence of Bacterial Vaginosis: A Review. J. Low. Genit. Tract. Dis. 2014;18:79–86. doi: 10.1097/LGT.0b013e31829156ec. [DOI] [PubMed] [Google Scholar]
- 118.Moludi J., Kafil H.S., Qaisar S.A., Gholizadeh P., Alizadeh M., Vayghyan H.J. Effect of Probiotic Supplementation along with Calorie Restriction on Metabolic Endotoxemia, and Inflammation Markers in Coronary Artery Disease Patients: A Double Blind Placebo Controlled Randomized Clinical Trial. Nutr. J. 2021;20:47. doi: 10.1186/s12937-021-00703-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang B., Yue Y., Chen Y., Ding M., Li B., Wang L., Wang Q., Stanton C., Ross R.P., Zhao J., et al. Lactobacillus Plantarum CCFM1143 Alleviates Chronic Diarrhea via Inflammation Regulation and Gut Microbiota Modulation: A Double-Blind, Randomized, Placebo-Controlled Study. Front. Immunol. 2021;12:746585. doi: 10.3389/fimmu.2021.746585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gamallat Y., Meyiah A., Kuugbee E.D., Hago A.M., Chiwala G., Awadasseid A., Bamba D., Zhang X., Shang X., Luo F., et al. Lactobacillus Rhamnosus Induced Epithelial Cell Apoptosis, Ameliorates Inflammation and Prevents Colon Cancer Development in an Animal Model. Biomed. Pharmacother. 2016;83:536–541. doi: 10.1016/j.biopha.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 121.Kuugbee E.D., Shang X., Gamallat Y., Bamba D., Awadasseid A., Suliman M.A., Zang S., Ma Y., Chiwala G., Xin Y., et al. Structural Change in Microbiota by a Probiotic Cocktail Enhances the Gut Barrier and Reduces Cancer via TLR2 Signaling in a Rat Model of Colon Cancer. Dig. Dis. Sci. 2016;61:2908–2920. doi: 10.1007/s10620-016-4238-7. [DOI] [PubMed] [Google Scholar]
- 122.Boudeau J., Glasser A.-L., Julien S., Colombel J.-F., Darfeuille-Michaud A. Inhibitory Effect of Probiotic Escherichia Coli Strain Nissle 1917 on Adhesion to and Invasion of Intestinal Epithelial Cells by Adherent-Invasive, E. Coli Strains Isolated from Patients with Crohn’s Disease: INHIBITORY EFFECT OF PROBIOTIC E. COLI NISSLE 1917 ON AIEC COLONIZATION. Aliment. Pharmacol. Ther. 2003;18:45–56. doi: 10.1046/j.1365-2036.2003.01638.x. [DOI] [PubMed] [Google Scholar]
- 123.Kelesidis T., Pothoulakis C. Efficacy and Safety of the Probiotic Saccharomyces Boulardii for the Prevention and Therapy of Gastrointestinal Disorders. Ther. Adv. Gastroenterol. 2012;5:111–125. doi: 10.1177/1756283X11428502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yan F., Li N., Shi J., Li H., Yue Y., Jiao W., Wang N., Song Y., Huo G., Li B. Lactobacillus Acidophilus Alleviates Type 2 Diabetes by Regulating Hepatic Glucose, Lipid Metabolism and Gut Microbiota in Mice. Food Funct. 2019;10:5804–5815. doi: 10.1039/C9FO01062A. [DOI] [PubMed] [Google Scholar]
- 125.Yang B., Huang Z., He Z., Yue Y., Zhou Y., Ross R.P., Stanton C., Zhang H., Zhao J., Chen W. Protective Effect of Bifidobacterium Bifidum FSDJN7O5 and Bifidobacterium Breve FHNFQ23M3 on Diarrhea Caused by Enterotoxigenic Escherichia Coli. Food Funct. 2021;12:7271–7282. doi: 10.1039/D1FO00504A. [DOI] [PubMed] [Google Scholar]
- 126.Sullivan Å., Bennet R., Viitanen M., Palmgren A.-C., Nord C.E. Influence of Lactobacillus F19 on Intestinal Microflora in Children and Elderly Persons and Impact on Helicobacter Pylori Infections. Microb. Ecol. Health Dis. 2002;14:17–21. doi: 10.1080/089106002760003305. [DOI] [Google Scholar]
- 127.Hlivak P., Odraska J., Ferencik M., Ebringer L., Jahnova E., Mikes Z. One-Year Application of Probiotic Strain Enterococcus Faecium M-74 Decreases Serum Cholesterol Levels. Bratisl Lek Listy. 2005;106:67–72. [PubMed] [Google Scholar]
- 128.Pakdaman M.N., Udani J.K., Molina J.P., Shahani M. The Effects of the DDS-1 Strain of Lactobacillus on Symptomatic Relief for Lactose Intolerance - a Randomized, Double-Blind, Placebo-Controlled, Crossover Clinical Trial. Nutr. J. 2015;15:56. doi: 10.1186/s12937-016-0172-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lata J., Novotný I., Příbramská V., Juránková J., Frič P., Kroupa R., Stibůrek O. The Effect of Probiotics on Gut Flora, Level of Endotoxin and Child–Pugh Score in Cirrhotic Patients: Results of a Double-Blind Randomized Study. Eur. J. Gastroenterol. Hepatol. 2007;19:1111–1113. doi: 10.1097/MEG.0b013e3282efa40e. [DOI] [PubMed] [Google Scholar]
- 130.Lunia M.K., Sharma B.C., Sharma P., Sachdeva S., Srivastava S. Probiotics Prevent Hepatic Encephalopathy in Patients with Cirrhosis: A Randomized Controlled Trial. Clin. Gastroenterol. Hepatol. 2014;12:1003–1008.e1. doi: 10.1016/j.cgh.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 131.Zhu G., Ma F., Wang G., Wang Y., Zhao J., Zhang H., Chen W. Bifidobacteria Attenuate the Development of Metabolic Disorders, with Inter- and Intra-Species Differences. Food Funct. 2018;9:3509–3522. doi: 10.1039/C8FO00100F. [DOI] [PubMed] [Google Scholar]
- 132.Sohn M., Na G.Y., Chu J., Joung H., Kim B.-K., Lim S. Efficacy and Safety of Lactobacillus Plantarum K50 on Lipids in Koreans with Obesity: A Randomized, Double-Blind Controlled Clinical Trial. Front. Endocrinol. 2022;12:790046. doi: 10.3389/fendo.2021.790046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Anukam K.C., Hayes K., Summers K., Reid G. Probiotic Lactobacillus Rhamnosus GR-1 and Lactobacillus Reuteri RC-14 May Help Downregulate TNF-Alpha, IL-6, IL-8, IL-10 and IL-12 (P70) in the Neurogenic Bladder of Spinal Cord Injured Patient with Urinary Tract Infections: A Two-Case Study. Adv. Urol. 2009;2009:1–5. doi: 10.1155/2009/680363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sivamaruthi B.S., Kesika P., Chaiyasut C. A review on anti-aging properties of probiotics. Int. J. App. Pharm. 2018;10:23. doi: 10.22159/ijap.2018v10i5.28249. [DOI] [Google Scholar]
- 135.Salazar N., Valdés-Varela L., González S., Gueimonde M., de los Reyes-Gavilán C.G. Nutrition and the Gut Microbiome in the Elderly. Gut Microbes. 2017;8:82–97. doi: 10.1080/19490976.2016.1256525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lahtinen S.J., Tammela L., Korpela J., Parhiala R., Ahokoski H., Mykkänen H., Salminen S.J. Probiotics Modulate the Bifidobacterium Microbiota of Elderly Nursing Home Residents. AGE. 2009;31:59–66. doi: 10.1007/s11357-008-9081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rampelli S., Candela M., Severgnini M., Biagi E., Turroni S., Roselli M., Carnevali P., Donini L., Brigidi P. A Probiotics-Containing Biscuit Modulates the Intestinal Microbiota in the Elderly. J. Nutr. Health Aging. 2013;17:166–172. doi: 10.1007/s12603-012-0372-x. [DOI] [PubMed] [Google Scholar]
- 138.Valentini L., Pinto A., Bourdel-Marchasson I., Ostan R., Brigidi P., Turroni S., Hrelia S., Hrelia P., Bereswill S., Fischer A., et al. Impact of Personalized Diet and Probiotic Supplementation on Inflammation, Nutritional Parameters and Intestinal Microbiota—The “RISTOMED Project”: Randomized Controlled Trial in Healthy Older People. Clin. Nutr. 2015;34:593–602. doi: 10.1016/j.clnu.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 139.Ahmed M., Prasad J., Gill H., Stevenson L., Gopal P. Impact of Consumption of Different Levels of Bifidobacterium Lactis HN019 on the Intestinal Microflora of Elderly Human Subjects. J. Nutr. Health Aging. 2007;11:26–31. [PubMed] [Google Scholar]
- 140.Azad M.A.K., Sarker M., Wan D. Immunomodulatory Effects of Probiotics on Cytokine Profiles. BioMed Res. Int. 2018;2018:8063647. doi: 10.1155/2018/8063647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.María Remes-Troche J., Coss-Adame E., Ángel Valdovinos-Díaz M., Gómez-Escudero O., Eugenia Icaza-Chávez M., Antonio Chávez-Barrera J., Zárate-Mondragón F., Antonio Velarde-Ruíz Velasco J., Rafael Aceves-Tavares G., Antonio Lira-Pedrín M., et al. Lactobacillus Acidophilus LB: A Useful Pharmabiotic for the Treatment of Digestive Disorders. Ther. Adv. Gastroenterol. 2020;13:175628482097120. doi: 10.1177/1756284820971201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sánchez B., Delgado S., Blanco-Míguez A., Lourenço A., Gueimonde M., Margolles A. Probiotics, Gut Microbiota, and Their Influence on Host Health and Disease. Mol. Nutr. Food Res. 2017;61:1600240. doi: 10.1002/mnfr.201600240. [DOI] [PubMed] [Google Scholar]
- 143.Hemarajata P., Versalovic J. Effects of Probiotics on Gut Microbiota: Mechanisms of Intestinal Immunomodulation and Neuromodulation. Ther. Adv. Gastroenterol. 2013;6:39–51. doi: 10.1177/1756283X12459294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen L., Li H., Li J., Chen Y., Yang Y. Lactobacillus Rhamnosus GG Treatment Improves Intestinal Permeability and Modulates Microbiota Dysbiosis in an Experimental Model of Sepsis. Int. J. Mol. Med. 2019 doi: 10.3892/ijmm.2019.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Forsyth C.B., Farhadi A., Jakate S.M., Tang Y., Shaikh M., Keshavarzian A. Lactobacillus GG Treatment Ameliorates Alcohol-Induced Intestinal Oxidative Stress, Gut Leakiness, and Liver Injury in a Rat Model of Alcoholic Steatohepatitis. Alcohol. 2009;43:163–172. doi: 10.1016/j.alcohol.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tao Y., Drabik K.A., Waypa T.S., Musch M.W., Alverdy J.C., Schneewind O., Chang E.B., Petrof E.O. Soluble Factors from Lactobacillus GG Activate MAPKs and Induce Cytoprotective Heat Shock Proteins in Intestinal Epithelial Cells. Am. J. Physiol.-Cell Physiol. 2006;290:C1018–C1030. doi: 10.1152/ajpcell.00131.2005. [DOI] [PubMed] [Google Scholar]
- 147.Park J.-S., Choi J.W., Jhun J., Kwon J.Y., Lee B.-I., Yang C.W., Park S.-H., Cho M.-L. Lactobacillus Acidophilus Improves Intestinal Inflammation in an Acute Colitis Mouse Model by Regulation of Th17 and Treg Cell Balance and Fibrosis Development. J. Med. Food. 2018;21:215–224. doi: 10.1089/jmf.2017.3990. [DOI] [PubMed] [Google Scholar]
- 148.Vemuri R., Shinde T., Gundamaraju R., Gondalia S., Karpe A., Beale D., Martoni C., Eri R. Lactobacillus Acidophilus DDS-1 Modulates the Gut Microbiota and Improves Metabolic Profiles in Aging Mice. Nutrients. 2018;10:1255. doi: 10.3390/nu10091255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li X., Huang Y., Song L., Xiao Y., Lu S., Xu J., Li J., Ren Z. Lactobacillus Plantarum Prevents Obesity via Modulation of Gut Microbiota and Metabolites in High-Fat Feeding Mice. J. Funct. Foods. 2020;73:104103. doi: 10.1016/j.jff.2020.104103. [DOI] [Google Scholar]
- 150.Graziani C., Petito V., Del Chierico F., Mangiola F., Pecere S., Schiavoni E., Pizzoferrato M., Lopetuso L.R., Putignani L., Gasbarrini A., et al. P115 Escherichia Coli Nissle 1917 Modulate Gut Microbiota Composition in Ulcerative Colitis Patients. J. Crohn’s Colitis. 2017;11:S133–S134. doi: 10.1093/ecco-jcc/jjx002.241. [DOI] [Google Scholar]
- 151.Schlee M., Wehkamp J., Altenhoefer A., Oelschlaeger T.A., Stange E.F., Fellermann K. Induction of Human β-Defensin 2 by the Probiotic Escherichia Coli Nissle 1917 Is Mediated through Flagellin. Infect. Immun. 2007;75:2399–2407. doi: 10.1128/IAI.01563-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Everard A., Matamoros S., Geurts L., Delzenne N.M., Cani P.D. Saccharomyces Boulardii Administration Changes Gut Microbiota and Reduces Hepatic Steatosis, Low-Grade Inflammation, and Fat Mass in Obese and Type 2 Diabetic Db/Db Mice. mBio. 2014;5:e01011–e01014. doi: 10.1128/mBio.01011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Grander C., Adolph T.E., Wieser V., Lowe P., Wrzosek L., Gyongyosi B., Ward D.V., Grabherr F., Gerner R.R., Pfister A., et al. Recovery of Ethanol-Induced Akkermansia Muciniphila Depletion Ameliorates Alcoholic Liver Disease. Gut. 2018;67:891–901. doi: 10.1136/gutjnl-2016-313432. [DOI] [PubMed] [Google Scholar]
- 154.O’Toole P.W., Marchesi J.R., Hill C. Next-Generation Probiotics: The Spectrum from Probiotics to Live Biotherapeutics. Nat. Microbiol. 2017;2:17057. doi: 10.1038/nmicrobiol.2017.57. [DOI] [PubMed] [Google Scholar]
- 155.Yao M., Qv L., Lu Y., Wang B., Berglund B., Li L. An Update on the Efficacy and Functionality of Probiotics for the Treatment of Non-Alcoholic Fatty Liver Disease. Engineering. 2021;7:679–686. doi: 10.1016/j.eng.2020.01.017. [DOI] [Google Scholar]
- 156.Roh Y.S., Seki E. Toll-like Receptors in Alcoholic Liver Disease, Non-Alcoholic Steatohepatitis and Carcinogenesis: The Role of TLR in ALD, NASH and HCC. J. Gastroenterol. Hepatol. 2013;28:38–42. doi: 10.1111/jgh.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang W., Shi L.P., Shi L., Xu L. Efficacy of probiotics on the treatment of non-alcoholic fatty liver disease. Zhonghua Nei Ke Za Zhi. 2018;57:101–106. doi: 10.3760/cma.j.issn.0578-1426.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 158.Wieland A., Frank D.N., Harnke B., Bambha K. Systematic review: Microbial dysbiosis and nonalcoholic fatty liver disease. Aliment. Pharm. 2015;42:1051–1063. doi: 10.1111/apt.13376. [DOI] [PubMed] [Google Scholar]
- 159.Mantovani A., Dalbeni A. Treatments for NAFLD: State of Art. Int. J. Mol. Sci. 2021;22:2350. doi: 10.3390/ijms22052350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Borrelli A., Bonelli P., Tuccillo F.M., Goldfine I.D., Evans J.L., Buonaguro F.M., Mancini A. Role of Gut Microbiota and Oxidative Stress in the Progression of Non-Alcoholic Fatty Liver Disease to Hepatocarcinoma: Current and Innovative Therapeutic Approaches. Redox Biol. 2018;15:467–479. doi: 10.1016/j.redox.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Perumpail B., Li A., John N., Sallam S., Shah N., Kwong W., Cholankeril G., Kim D., Ahmed A. The Therapeutic Implications of the Gut Microbiome and Probiotics in Patients with NAFLD. Diseases. 2019;7:27. doi: 10.3390/diseases7010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lei K., Li Y., Wang Y., Wen J., Wu H., Yu D., Li W. Effect of Dietary Supplementation of Bacillus Subtilis B10 on Biochemical and Molecular Parameters in the Serum and Liver of High-Fat Diet-Induced Obese Mice. J. Zhejiang Univ. Sci. B. 2015;16:487–495. doi: 10.1631/jzus.B1400342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Vajro P., Mandato C., Veropalumbo C., De Micco I. Probiotics: A Possible Role in Treatment of Adult and Pediatric Non Alcoholic Fatty Liver Disease. Ann. Hepatol. 2013;12:161–163. doi: 10.1016/S1665-2681(19)31401-2. [DOI] [PubMed] [Google Scholar]
- 164.Li C., Nie S.-P., Zhu K.-X., Ding Q., Li C., Xiong T., Xie M.-Y. Lactobacillus Plantarum NCU116 Improves Liver Function, Oxidative Stress and Lipid Metabolism in Rats with High Fat Diet Induced Non-Alcoholic Fatty Liver Disease. Food Funct. 2014;5:3216–3223. doi: 10.1039/C4FO00549J. [DOI] [PubMed] [Google Scholar]
- 165.Zhao Z., Wang C., Zhang L., Zhao Y., Duan C., Zhang X., Gao L., Li S. Lactobacillus Plantarum NA136 Improves the Non-Alcoholic Fatty Liver Disease by Modulating the AMPK/Nrf2 Pathway. Appl. Microbiol. Biotechnol. 2019;103:5843–5850. doi: 10.1007/s00253-019-09703-4. [DOI] [PubMed] [Google Scholar]
- 166.Xin J., Zeng D., Wang H., Ni X., Yi D., Pan K., Jing B. Preventing Non-Alcoholic Fatty Liver Disease through Lactobacillus Johnsonii BS15 by Attenuating Inflammation and Mitochondrial Injury and Improving Gut Environment in Obese Mice. Appl. Microbiol. Biotechnol. 2014;98:6817–6829. doi: 10.1007/s00253-014-5752-1. [DOI] [PubMed] [Google Scholar]
- 167.Ritze Y., Bárdos G., Claus A., Ehrmann V., Bergheim I., Schwiertz A., Bischoff S.C. Lactobacillus Rhamnosus GG Protects against Non-Alcoholic Fatty Liver Disease in Mice. PLoS ONE. 2014;9:e80169. doi: 10.1371/journal.pone.0080169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ren T., Huang C., Cheng M. Dietary Blueberry and Bifidobacteria Attenuate Nonalcoholic Fatty Liver Disease in Rats by Affecting SIRT1-Mediated Signaling Pathway. Oxidative Med. Cell. Longev. 2014;2014:1–12. doi: 10.1155/2014/469059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Xu R., Wan Y., Fang Q., Lu W., Cai W. Supplementation with Probiotics Modifies Gut Flora and Attenuates Liver Fat Accumulation in Rat Nonalcoh.holic Fatty Liver Disease Model. J. Clin. Biochem. Nutr. 2011;50:72–77. doi: 10.3164/jcbn.11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bubnov R.V., Babenko L.P., Lazarenko L.M., Mokrozub V.V., Demchenko O.A., Nechypurenko O.V., Spivak M.Y. Comparative Study of Probiotic Effects of Lactobacillus and Bifidobacteria Strains on Cholesterol Levels, Liver Morphology and the Gut Microbiota in Obese Mice. EPMA J. 2017;8:357–376. doi: 10.1007/s13167-017-0117-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Xue L., He J., Gao N., Lu X., Li M., Wu X., Liu Z., Jin Y., Liu J., Xu J., et al. Probiotics May Delay the Progression of Nonalcoholic Fatty Liver Disease by Restoring the Gut Microbiota Structure and Improving Intestinal Endotoxemia. Sci. Rep. 2017;7:45176. doi: 10.1038/srep45176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Seo M., Inoue I., Tanaka M., Matsuda N., Nakano T., Awata T., Katayama S., Alpers D.H., Komoda T. Clostridium Butyricum MIYAIRI 588 Improves High-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease in Rats. Dig. Dis. Sci. 2013;58:3534–3544. doi: 10.1007/s10620-013-2879-3. [DOI] [PubMed] [Google Scholar]
- 173.Ma X., Hua J., Li Z. Probiotics Improve High Fat Diet-Induced Hepatic Steatosis and Insulin Resistance by Increasing Hepatic NKT Cells. J. Hepatol. 2008;49:821–830. doi: 10.1016/j.jhep.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Li Z. Probiotics and Antibodies to TNF Inhibit Inflammatory Activity and Improve Nonalcoholic Fatty Liver Disease. Hepatology. 2003;37:343–350. doi: 10.1053/jhep.2003.50048. [DOI] [PubMed] [Google Scholar]
- 175.Munukka E., Rintala A., Toivonen R., Nylund M., Yang B., Takanen A., Hänninen A., Vuopio J., Huovinen P., Jalkanen S., et al. Faecalibacterium Prausnitzii Treatment Improves Hepatic Health and Reduces Adipose Tissue Inflammation in High-Fat Fed Mice. ISME J. 2017;11:1667–1679. doi: 10.1038/ismej.2017.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ahn S.B., Jun D.W., Kang B.-K., Lim J.H., Lim S., Chung M.-J. Randomized, Double-Blind, Placebo-Controlled Study of a Multispecies Probiotic Mixture in Nonalcoholic Fatty Liver Disease. Sci Rep. 2019;9:5688. doi: 10.1038/s41598-019-42059-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chen Y., Feng R., Yang X., Dai J., Huang M., Ji X., Li Y., Okekunle A.P., Gao G., Onwuka J.U., et al. Yogurt Improves Insulin Resistance and Liver Fat in Obese Women with Nonalcoholic Fatty Liver Disease and Metabolic Syndrome: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2019;109:1611–1619. doi: 10.1093/ajcn/nqy358. [DOI] [PubMed] [Google Scholar]
- 178.Sepideh A., Karim P., Hossein A., Leila R., Hamdollah M., Mohammad E.G., Mojtaba S., Mohammad S., Ghader G., Seyed Moayed A. Effects of Multistrain Probiotic Supplementation on Glycemic and Inflammatory Indices in Patients with Nonalcoholic Fatty Liver Disease: A Double-Blind Randomized Clinical Trial. J. Am. Coll. Nutr. 2016;35:500–505. doi: 10.1080/07315724.2015.1031355. [DOI] [PubMed] [Google Scholar]
- 179.Aller R., De Luis D.A., Izaola O., Conde R., Gonzalez Sagrado M., Primo D., De La Fuente B., Gonzalez J. Effect of a Probiotic on Liver Aminotransferases in Nonalcoholic Fatty Liver Disease Patients: A Double Blind Randomized Clinical Trial. Eur. Rev. Med. Pharm. Sci. 2011;15:1090–1095. [PubMed] [Google Scholar]
- 180.Derosa G., Guasti L., D’Angelo A., Martinotti C., Valentino M.C., Di Matteo S., Bruno G.M., Maresca A.M., Gaudio G.V., Maffioli P. Probiotic Therapy with VSL#3® in Patients with NAFLD: A Randomized Clinical Trial. Front. Nutr. 2022;9:846873. doi: 10.3389/fnut.2022.846873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Shavakhi A., Minakari M., Firouzian H., Assali R., Hekmatdoost A., Ferns G. Effect of a Probiotic and Metformin on Liver Aminotransferases in Non-Alcoholic Steatohepatitis: A Double Blind Randomized Clinical Trial. Int. J. Prev. Med. 2013;4:531–537. [PMC free article] [PubMed] [Google Scholar]
- 182.Kobyliak N., Abenavoli L., Mykhalchyshyn G., Kononenko L., Boccuto L., Kyriienko D., Dynnyk O. A Multi-Strain Probiotic Reduces the Fatty Liver Index, Cytokines and Aminotransferase Levels in NAFLD Patients: Evidence from a Randomized Clinical Trial. JGLD. 2018;27:41–49. doi: 10.15403/jgld.2014.1121.271.kby. [DOI] [PubMed] [Google Scholar]
- 183.Mei L., Tang Y., Li M., Yang P., Liu Z., Yuan J., Zheng P. Co-Administration of Cholesterol-Lowering Probiotics and Anthraquinone from Cassia Obtusifolia, L. Ameliorate Non-Alcoholic Fatty Liver. PLoS ONE. 2015;10:e0138078. doi: 10.1371/journal.pone.0138078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Park E.-J., Lee Y.-S., Kim S.M., Park G.-S., Lee Y.H., Jeong D.Y., Kang J., Lee H.-J. Beneficial Effects of Lactobacillus Plantarum Strains on Non-Alcoholic Fatty Liver Disease in High Fat/High Fructose Diet-Fed Rats. Nutrients. 2020;12:542. doi: 10.3390/nu12020542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yan Y., Liu C., Zhao S., Wang X., Wang J., Zhang H., Wang Y., Zhao G. Probiotic Bifidobacterium Lactis V9 Attenuates Hepatic Steatosis and Inflammation in Rats with Non-Alcoholic Fatty Liver Disease. AMB Expr. 2020;10:101. doi: 10.1186/s13568-020-01038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Nabavi S., Rafraf M., Somi M.H., Homayouni-Rad A., Asghari-Jafarabadi M. Effects of Probiotic Yogurt Consumption on Metabolic Factors in Individuals with Nonalcoholic Fatty Liver Disease. J. Dairy Sci. 2014;97:7386–7393. doi: 10.3168/jds.2014-8500. [DOI] [PubMed] [Google Scholar]
- 187.Duseja A., Acharya S.K., Mehta M., Chhabra S., Shalimar R.S., Das A., Dattagupta S., Dhiman R.K., Chawla Y.K. High Potency Multistrain Probiotic Improves Liver Histology in Non-Alcoholic Fatty Liver Disease (NAFLD): A Randomised, Double-Blind, Proof of Concept Study. BMJ Open Gastroenterol. 2019;6:e000315. doi: 10.1136/bmjgast-2019-000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ma J., Zhou Q., Li H. Gut Microbiota and Nonalcoholic Fatty Liver Disease: Insights on Mechanisms and Therapy. Nutrients. 2017;9:1124. doi: 10.3390/nu9101124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Fianchi F., Liguori A., Gasbarrini A., Grieco A., Miele L. Nonalcoholic Fatty Liver Disease (NAFLD) as Model of Gut–Liver Axis Interaction: From Pathophysiology to Potential Target of Treatment for Personalized Therapy. Int. J. Mol. Sci. 2021;22:6485. doi: 10.3390/ijms22126485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Gibson G.R., Roberfroid M.B. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J. Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 191.Holscher H.D. Dietary Fiber and Prebiotics and the Gastrointestinal Microbiota. Gut Microbes. 2017;8:172–184. doi: 10.1080/19490976.2017.1290756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.de Vrese M., Schrezenmeir J. Probiotics, Prebiotics, and Synbiotics. In: Stahl U., Donalies U.E.B., Nevoigt E., editors. Food Biotechnology. Vol. 111. Springer; Berlin/Heidelberg, Germany: 2008. pp. 1–66. Advances in Biochemical Engineering/Biotechnology. [DOI] [PubMed] [Google Scholar]
- 193.Chambers E.S., Byrne C.S., Morrison D.J., Murphy K.G., Preston T., Tedford C., Garcia-Perez I., Fountana S., Serrano-Contreras J.I., Holmes E., et al. Dietary Supplementation with Inulin-Propionate Ester or Inulin Improves Insulin Sensitivity in Adults with Overweight and Obesity with Distinct Effects on the Gut Microbiota, Plasma Metabolome and Systemic Inflammatory Responses: A Randomised Cross-over Trial. Gut. 2019;68:1430–1438. doi: 10.1136/gutjnl-2019-318424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tandon D., Haque M.M., Gote M., Jain M., Bhaduri A., Dubey A.K., Mande S.S. A Prospective Randomized, Double-Blind, Placebo-Controlled, Dose-Response Relationship Study to Investigate Efficacy of Fructo-Oligosaccharides (FOS) on Human Gut Microflora. Sci. Rep. 2019;9:5473. doi: 10.1038/s41598-019-41837-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Liu F., Li P., Chen M., Luo Y., Prabhakar M., Zheng H., He Y., Qi Q., Long H., Zhang Y., et al. Fructooligosaccharide (FOS) and Galactooligosaccharide (GOS) Increase Bifidobacterium but Reduce Butyrate Producing Bacteria with Adverse Glycemic Metabolism in Healthy Young Population. Sci. Rep. 2017;7:11789. doi: 10.1038/s41598-017-10722-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Parnell J.A., Raman M., Rioux K.P., Reimer R.A. The Potential Role of Prebiotic Fibre for Treatment and Management of Non-Alcoholic Fatty Liver Disease and Associated Obesity and Insulin Resistance. Liver Int. 2012;32:701–711. doi: 10.1111/j.1478-3231.2011.02730.x. [DOI] [PubMed] [Google Scholar]
- 197.Daubioul C.A., Horsmans Y., Lambert P., Danse E., Delzenne N.M. Effects of Oligofructose on Glucose and Lipid Metabolism in Patients with Nonalcoholic Steatohepatitis: Results of a Pilot Study. Eur. J. Clin. Nutr. 2005;59:723–726. doi: 10.1038/sj.ejcn.1602127. [DOI] [PubMed] [Google Scholar]
- 198.Fan J.-G. Effect of Lactulose on Establishment of a Rat Non-Alcoholic Steatohepatitis Model. WJG. 2005;11:5053. doi: 10.3748/wjg.v11.i32.5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Matsumoto K., Ichimura M., Tsuneyama K., Moritoki Y., Tsunashima H., Omagari K., Hara M., Yasuda I., Miyakawa H., Kikuchi K. Fructo-Oligosaccharides and Intestinal Barrier Function in a Methionine–Choline-Deficient Mouse Model of Nonalcoholic Steatohepatitis. PLoS ONE. 2017;12:e0175406. doi: 10.1371/journal.pone.0175406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ebrahimi-Mameghani M., Aliashrafi S., Javadzadeh Y., AsghariJafarabadi M. The Effect of Chlorella Vulgaris Supplementation on Liver Enzymes, Serum Glucose and Lipid Profile in Patients with Non-Alcoholic Fatty Liver Disease. Health Promot. Perspect. 2014 doi: 10.5681/HPP.2014.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Javadi L., Ghavami M., Khoshbaten M., Safaiyan A., Barzegari A., Pourghassem Gargari B. The Effect of Probiotic and/or Prebiotic on Liver Function Tests in Patients with Nonalcoholic Fatty Liver Disease: A Double Blind Randomized Clinical Trial. Iran. Red Crescent. Med. J. 2017;19 doi: 10.5812/ircmj.46017. [DOI] [Google Scholar]
- 202.Vulevic J., Juric A., Walton G.E., Claus S.P., Tzortzis G., Toward R.E., Gibson G.R. Influence of Galacto-Oligosaccharide Mixture (B-GOS) on Gut Micro.obiota, Immune Parameters and Metabonomics in Elderly Persons. Br. J. Nutr. 2015;114:586–595. doi: 10.1017/S0007114515001889. [DOI] [PubMed] [Google Scholar]
- 203.Vulevic J., Drakoularakou A., Yaqoob P., Tzortzis G., Gibson G.R. Modulation of the Fecal Microflora Profile and Immune Function by a Novel Trans-Galactooligosaccharide Mixture (B-GOS) in Healthy Elderly Volunteers. Am. J. Clin. Nutr. 2008;88:1438–1446. doi: 10.3945/ajcn.2008.26242. [DOI] [PubMed] [Google Scholar]
- 204.Walton G.E., van den Heuvel E.G.H.M., Kosters M.H.W., Rastall R.A., Tuohy K.M., Gibson G.R. A Randomised Crossover Study Investigating the Effects of Galacto-Oligosaccharides on the Faecal Microbiota in Men and Women over 50 Years of Age. Br. J. Nutr. 2012;107:1466–1475. doi: 10.1017/S0007114511004697. [DOI] [PubMed] [Google Scholar]
- 205.Markowiak P., Śliżewska K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients. 2017;9:1021. doi: 10.3390/nu9091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Malaguarnera M., Vacante M., Antic T., Giordano M., Chisari G., Acquaviva R., Mastrojeni S., Malaguarnera G., Mistretta A., Li Volti G., et al. Bifidobacterium Longum with Fructo-Oligosaccharides in Patients with Non Alcoholic Steatohepatitis. Dig. Dis. Sci. 2012;57:545–553. doi: 10.1007/s10620-011-1887-4. [DOI] [PubMed] [Google Scholar]
- 207.Eslamparast T., Poustchi H., Zamani F., Sharafkhah M., Malekzadeh R., Hekmatdoost A. Synbiotic Supplementation in Nonalcoholic Fatty Liver Disease: A Randomized, Double-Blind, Placebo-Controlled Pilot Study. Am. J. Clin. Nutr. 2014;99:535–542. doi: 10.3945/ajcn.113.068890. [DOI] [PubMed] [Google Scholar]
- 208.Mofidi F., Poustchi H., Yari Z., Nourinayyer B., Merat S., Sharafkhah M., Malekzadeh R., Hekmatdoost A. Synbiotic Supplementation in Lean Patients with Non-Alcoholic Fatty Liver Disease: A Pilot, Randomised, Double-Blind, Placebo-Controlled, Clinical Trial. Br. J. Nutr. 2017;117:662–668. doi: 10.1017/S0007114517000204. [DOI] [PubMed] [Google Scholar]
- 209.Scorletti E., Afolabi P.R., Miles E.A., Smith D.E., Almehmadi A., Alshathry A., Childs C.E., Del Fabbro S., Bilson J., Moyses H.E., et al. Synbiotics Alter Fecal Microbiomes, But Not Liver Fat or Fibrosis, in a Randomized Trial of Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology. 2020;158:1597–1610.e7. doi: 10.1053/j.gastro.2020.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ferolla S., Couto C., Costa-Silva L., Armiliato G., Pereira C., Martins F., Ferrari M., Vilela E., Torres H., Cunha A., et al. Beneficial Effect of Synbiotic Supplementation on Hepatic Steatosis and Anthropometric Parameters, But Not on Gut Permeability in a Population with Nonalcoholic Steatohepatitis. Nutrients. 2016;8:397. doi: 10.3390/nu8070397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Asgharian A., Mohammadi V., Gholi Z., Esmaillzade A., Feizi A., Askari G. The Effect of Synbiotic Supplementation on Body Composition and Lipid Profile in Patients with NAFLD: A Randomized, Double Blind, Placebo-Controlled Clinical Trial Study. Iran. Red Crescent. Med. J. 2017;19 doi: 10.5812/ircmj.42902. [DOI] [Google Scholar]
- 212.Asgharian A., Askari G., Esmailzade A., Feizi A., Mohammadi V. The Effect of Symbiotic Supplementation on Liver Enzymes, c-Reactive Protein and Ultrasound Findings in Patients with Non-Alcoholic Fatty Liver Disease: A Clinical Trial. Int. J. Prev. Med. 2016;7:59. doi: 10.4103/2008-7802.178533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Björklund M., Ouwehand A.C., Forssten S.D., Nikkilä J., Tiihonen K., Rautonen N., Lahtinen S.J. Gut Microbiota of Healthy Elderly NSAID Users Is Selectively Modified with the Administration of Lactobacillus Acidophilus NCFM and Lactitol. AGE. 2012;34:987–999. doi: 10.1007/s11357-011-9294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Macfarlane S., Cleary S., Bahrami B., Reynolds N., Macfarlane G.T. Synbiotic Consumption Changes the Metabolism and Composition of the Gut Microbiota in Older People and Modifies Inflammatory Processes: A Randomised, Double-Blind, Placebo-Controlled Crossover Study. Aliment. Pharm. 2013;38:804–816. doi: 10.1111/apt.12453. [DOI] [PubMed] [Google Scholar]
- 215.Bartosch S., Woodmansey E.J., Paterson J.C.M., McMurdo M.E.T., Macfarlane G.T. Microbiological Effects of Consuming a Synbiotic Containing Bifidobacterium Bifidum, Bifidobacterium Lactis, and Oligofructose in Elderly Persons, Determined by Real-Time Polymerase Chain Reaction and Counting of Viable Bacteria. Clin. Infect. Dis. 2005;40:28–37. doi: 10.1086/426027. [DOI] [PubMed] [Google Scholar]
- 216.Juárez-Fernández M., Porras D., Petrov P., Román-Sagüillo S., García-Mediavilla M.V., Soluyanova P., Martínez-Flórez S., González-Gallego J., Nistal E., Jover R., et al. The Synbiotic Combination of Akkermansia Muciniphila and Quercetin Ameliorates Early Obesity and NAFLD through Gut Microbiota Reshaping and Bile Acid Metabolism Modulation. Antioxidants. 2021;10:2001. doi: 10.3390/antiox10122001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kim K.O., Gluck M. Fecal Microbiota Transplantation: An Update on Clinical Practice. Clin. Endosc. 2019;52:137–143. doi: 10.5946/ce.2019.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Cammarota G., Ianiro G., Gasbarrini A. Faecal Microbiota Transplantation in Clinical Practice. Gut. 2018;67:196.2–197. doi: 10.1136/gutjnl-2017-314049. [DOI] [PubMed] [Google Scholar]
- 219.Zhou D., Pan Q., Shen F., Cao H., Ding W., Chen Y., Fan J. Total Fecal Microbiota Transplantation Alleviates High-Fat Diet-Induced Steatohepatitis in Mice via Beneficial Regulation of Gut Microbiota. Sci. Rep. 2017;7:1529. doi: 10.1038/s41598-017-01751-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.García-Lezana T., Raurell I., Bravo M., Torres-Arauz M., Salcedo M.T., Santiago A., Schoenenberger A., Manichanh C., Genescà J., Martell M., et al. Restoration of a Healthy Intestinal Microbiota Normalizes Portal Hypertension in a Rat Model of Nonalcoholic Steatohepatitis: Liver Failure/Cirrhosis/Portal Hypertension. Hepatology. 2018;67:1485–1498. doi: 10.1002/hep.29646. [DOI] [PubMed] [Google Scholar]
- 221.Witjes J.J., Smits L.P., Pekmez C.T., Prodan A., Meijnikman A.S., Troelstra M.A., Bouter K.E.C., Herrema H., Levin E., Holleboom A.G., et al. Donor Fecal Microbiota Transplantation Alters Gut Microbiota and Metabolites in Obese Individuals with Steatohepatitis. Hepatol. Commun. 2020;4:1578–1590. doi: 10.1002/hep4.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Craven L., Rahman A., Nair Parvathy S., Beaton M., Silverman J., Qumosani K., Hramiak I., Hegele R., Joy T., Meddings J., et al. Allogenic Fecal Microbiota Transplantation in Patients with Nonalcoholic Fatty Liver Disease Improves Abnormal Small Intestinal Permeability: A Randomized Control Trial. Am. J. Gastroenterol. 2020;115:1055–1065. doi: 10.14309/ajg.0000000000000661. [DOI] [PubMed] [Google Scholar]