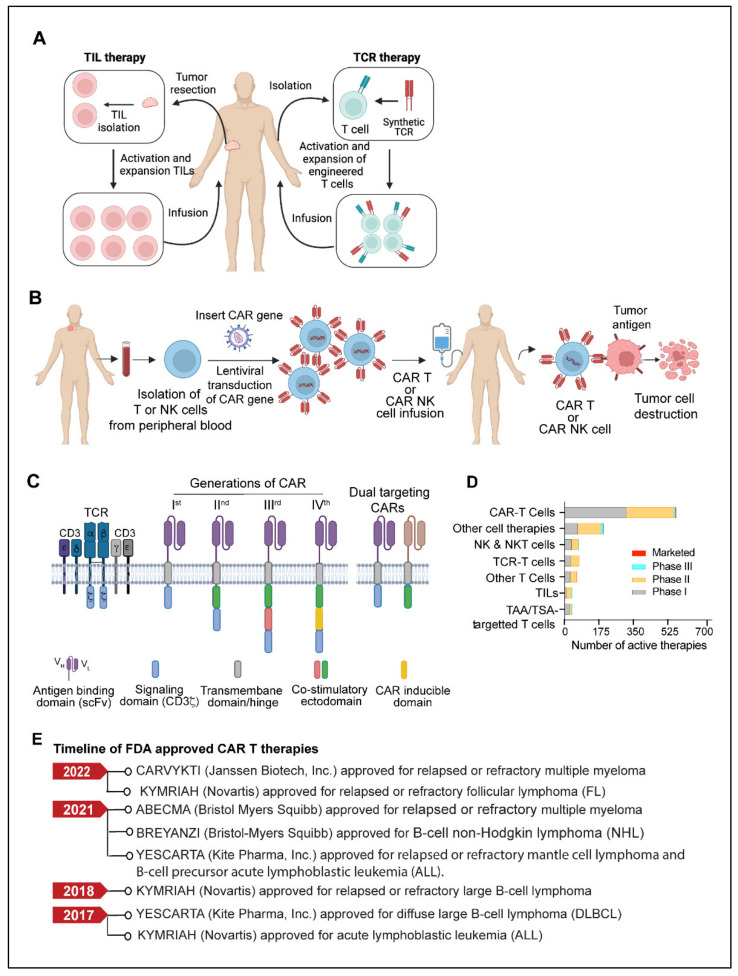
Figure 3.
(A) Model showing TIL therapy and TCR therapy. (B) Schematic outline of CAR T or CAR NK therapy. Briefly, T cells or NK cells are isolated from the apheresis blood of a cancer patient, followed by the lentiviral mediated introduction of the CAR gene. The CAR-expressing cells are expanded ex vivo and infused back into the patient’s body. (C) Model depicting CAR structure and generations of different CARs. An antigen-binding domain typically consists of variable heavy (VH) and light (VL) chains from a monoclonal antibody assembled through a linker sequence to form a single chain variable fragment (scFv). In first-generation CAR, the scFv is linked via a hinge and transmembrane domain to the CD3ζ, an intracellular T cell signaling domain of the T cell receptor. In the second and third-generation CARs, an additional one or two co-stimulatory domains are present. The fourth generation CARs are typically equipped with inducible domains A dual-targeting CAR contains two CARs, each targeting an independent antigen. (Created with BioRender.com) (D) Plots showing the preclinical and clinical trial status of different cell-based therapies for cancer treatment. (E) Timeline of U.S. FDA-approved CAR T therapies for various cancers. (Abbreviations: CAR, chimeric antigen receptor; NK, natural killer; TCR, T cell receptor; NKT, natural killer T cell; TAA, tumor-associated antigen; TSA, tumor-specific antigen; TIL, tumor-infiltrating lymphocytes; FDA, food and drug administration).