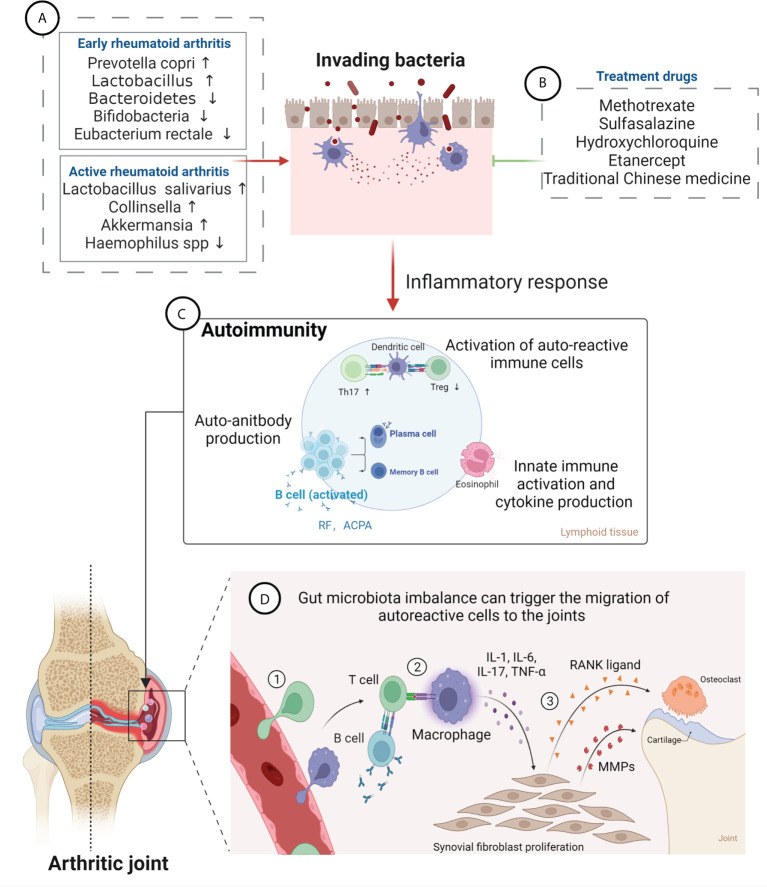
Figure 1.
Gut microbiota in the pathogenesis of rheumatoid arthritis (RA) and effects of RA therapeutic drugs on the gut microbiota. (A) Changes in the composition of gut microbiota at different stages of RA. Levels of Prevotella copri and Lactobacillus are increased, while those of Bacteroidetes, Bifidobacteria and Eubacterium rectale are decreased, at an early stage; Abundance of Lactobacillus salivarius, Collinsella, and Akkermansia is increased, while that of Haemophilus spp. is decreased, in the active RA phase. (B) RA treatment drugs can improve gut microbiota imbalance and relieve disease symptoms, mainly including methotrexate, sulfasalazine, hydroxychloroquine, etanercept, and traditional Chinese medicine. (C) Gut microbiota can lead to damage of the epithelium and to the opening of the paracellular pathway and can cross the epithelium and get in contact with the immune cells beneath the epithelial layer, which leads to inflammation. Furthermore, bacterial antigens promote activation of autoreactive immune cells (B cell and T cell) in the lymphoid tissues, resulting in an imbalance between regulatory T cells (Tregs) and T helper 17 (Th17) cells, leading to expansion of inflammatory response. Activated B cells produce autoantibodies (anti-citrullinated protein antibody and rheumatoid factor). (D) Gut microbiota imbalance can trigger the migration of autoreactive cells to the joints, causing cartilage and bone damage. ① Bacterial antigens trigger promotes inflammation in synovial membrane, attracting leukocytes into the tissue. ② Autoreactive cells activate macrophages, resulting in inflammatory cytokine production. ③ Cytokines induce fibroblasts to produce MMPs (matrix metalloproteinases) and RANKL (receptor activator of nuclear factor κB ligand), which mediate destruction of bone and cartilage tissue, leading to the development of RA.