Fig. 1.
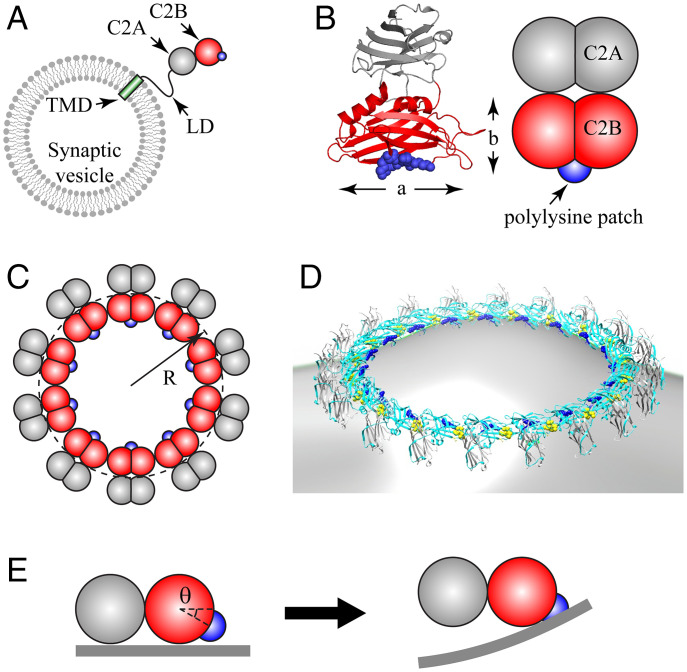
Coarse-grained model of Syt C2AB domain and oligomeric Syt rings. (A) Synaptic vesicles carry ∼15 copies of Syt, with each comprising a TMD, a flexible juxtamembrane LD, and the C2A and C2B globular domains (schematic, not to scale). The polylysine patch (blue) on C2B mediates Ca2+-independent binding to anionic membranes. (B) Crystal structure of Syt (PDB ID: 2R83) (Left) and our coarse-grained representation (Right). Each C2 domain is represented as two overlapping beads, radius , giving length nm and width nm. The positively charged polylysine patch (blue) on C2B is treated as a point charge. (C) Coarse-grained model representation of a Syt ring of radius R, Top view. (D) Rendition of a Syt ring bound to a membrane, based on EM reconstruction of Syt oligomers assembled on phospholipid monolayer tubes (43) (cyan, C2B; gray, C2A; blue, polylysine patch; yellow, Ca2+ binding loops on C2B). (E) C2AB subunit in a ring interacting with planar membrane (Side view). The polylysine patch lies below the plane of the ring, at an angle suggested by EM reconstruction, D. In the ring, the C2AB unit cannot rotate downward, so electrostatic attraction bends the charged membrane up toward the patch (Right).