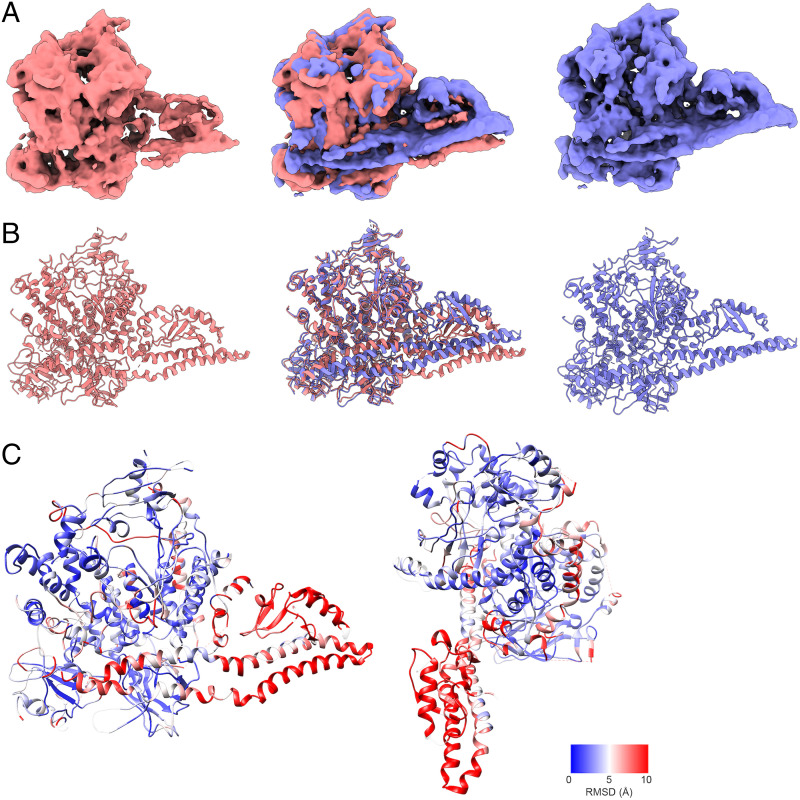
Fig. 4.
Three-dimensional variability analysis of the complex of PI3Kα with Nb3-159 shows extreme variability of the ABD and iSH2 domains. (A) The first component of the Nb3-159 3DVA resolves a motion from a structure similar to the consensus PI3Kα structure (blue) toward a structure with a radically deflected ABD and iSH2 domain (red), and overlay of these structures shows the degree of deflection (Middle). (B) Models of the two states of component 1 demonstrate a discontinuity in the iSH2 domain that cannot be explained without flexibility or kinking of the coiled-coil domain. (C) rmsd values calculated for the Cα positions show that the near edge of the iSH2 is deflected downward, while the far edge is less deflected. There are also changes in the kinase domain N lobes (773 to 777) and C lobes (864 to 874) as well as 803 to 811.