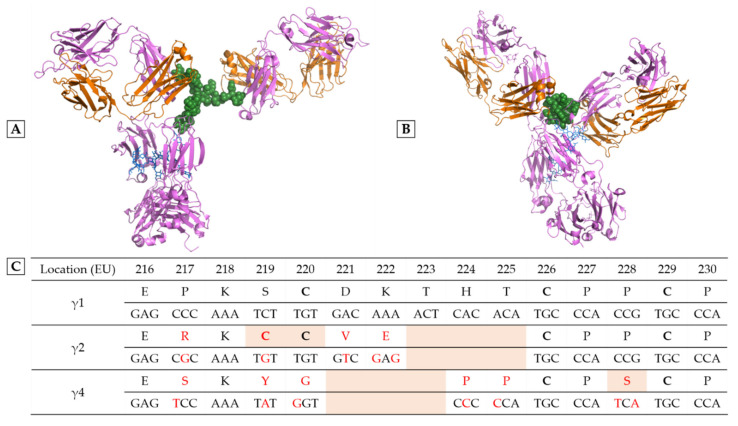
Figure 1.
Differences between human IgG subclasses in the hinge region. Structure of an IgG1 (A) and an IgG4 (B), the only two human IgG subclasses that have been crystallized in their entirety. The heavy chains are colored in pink, the light chains in orange; N-glycans are indicated in blue, and the hinge regions in green. Figures were elaborated using PyMOL Molecular Graphics System, version 1.7.4 (Schrödinger) from PDB file 1HZH representing the unique human IgG1 crystallized, named B12 and directed against the gp120 of HIV (human immunodeficiency virus) and from PDB file 5DK3 representing pembrolizumab, the only human IgG4 crystallized (G4e1 variant). The high flexibility of the IgG1 hinge region gives freedom to the Fab arms and causes strong asymmetry of the whole molecule. Unfortunately, there is no available structure of an entire human IgG2. (C) Hinge region alignment of heavy chains γ1, γ2, γ4. Amino acids (according to EU numbering) and nucleotides that differ to IgG1 sequence are colored in red. Cysteines engaged in disulfide bridge are in bold. Notable differences appear in orange background: 3 amino acids deletion in IgG2 and IgG4, cysteines 219 and 220 of IgG2, and serine 228 of IgG4 (see text).