Figure 8.
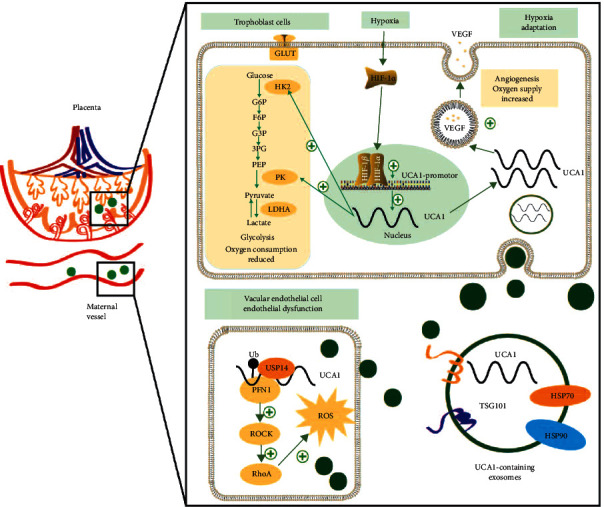
Graphic showing the mechanism of the human-specific UCA1-mediated adaptive response in preeclampsia. Because of failed remodeling, placental stress, and hypoxia, trophoblasts express HIF1α at high levels, which binds to the UCA1 promoter to upregulate its expression. In trophoblasts, UCA1 promotes the hypoxia adaptation by increasing VEGF secretion and the expression levels of PKM and HK2, both of which are key enzymes involved in glycolysis. Cytoplasmic UCA1 is packed into exosomes and transferred to vascular endothelial cells. In endothelial cells, UCA1 functions as a scaffold to recruit USP14 and PFN1, and prolong the half-life of the PFN1 protein, subsequently activating the RhoA/ROCK pathway to produce excess ROS and induce endothelial injury. In mothers, HIF1α-UCA1-induced adaptive responses may induce the clinical symptoms of preeclampsia.
