Figure 9.
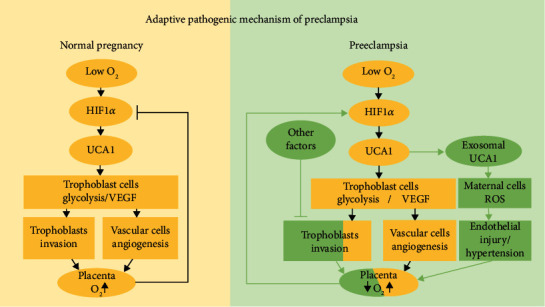
Graphic presenting the theory of HIF1α-induced adaptive responses in normal pregnancy and preeclampsia. (Left panel) In early gestation, trophoblasts are exposed to low oxygen levels and express HIF1α and UCA1 at high levels to regulate the trophoblast adaptation to hypoxia, including promoting glycolysis to reduce oxygen consumption and secreting VEGF to induce angiogenesis. With the deep invasion of trophoblasts and remodeling of spiral arteries, dilatation reduced the velocity of maternal blood into the intervillous space, and oxygen and nutrients are fully exchanged. Hence, HIF1α and UCA1 levels gradually decrease; this negative feedback loop is attenuated with the increase in gestational weeks in a normal pregnancy. (Right panel) With impaired trophoblast invasion and failed vessel remodeling caused by other factors, the transfer of oxygen and nutrients to the fetus and villous tree is decreased, leading to hypoxic and stressed placental conditions. The trophoblast cells express HIF1α and UCA1 at high levels again, promoting the local adaptation to hypoxia. However, these strategies do not completely compensate for sustained local hypoxia, and thus, trophoblasts secrete UCA1-containing exosomes that are transported to distant maternal cells and cause peripheral changes, including a series of manifestations characterized by hypertension, to partially relieve placental ischemia and hypoxia.
