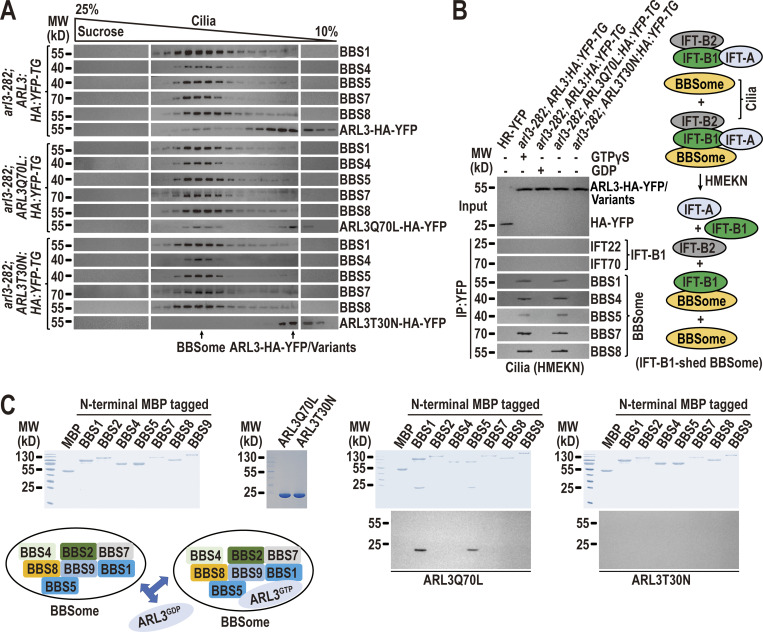
Figure 4.
The BBSome can shed from retrograde IFT trains at the TZ region to act as an ARL3 effector. (A) Immunoblots of sucrose density gradients of arl3-282; ARL3:HA:YFP-TG, arl3-282; ARL3Q70L:HA:YFP-TG, and arl3-282; ARL3T30N:HA:YFP-TG cilia probed with α-BBS1, α-BBS4, α-BBS5, α-BBS7, α-BBS8, and α-ARL3. (B) Immunoblots of α-YFP-captured proteins from HR-YFP (HA-YFP-expressing CC-125 cells), arl3-282; ARL3:HA:YFP-TG (in the presence of excessive GTPγS or GDP), arl3-282; ARL3Q70L:HA:YFP-TG, and arl3-282; ARL3T30N:HA:YFP-TG cilia probed for the IFT-B1 subunits IFT22 and IFT70 and the BBSome subunits BBS1, BBS4, BBS5, BBS7, and BBS8. HMEKN stands for the buffer used for solving cilia. Input was quantified with α-YFP by immunoblotting. A schematic representation of how a reservoir of the BBSome independent of IFT-B1 association exists in HMEKN buffer was shown on the right. (C) Bacterially expressed MBP, MBP-BBS1, MBP-BBS2, MBP-BBS4, MBP-BBS5, MBP-BBS7, MBP-BBS8, and MBP-BBS9 (left) were mixed with ARL3Q70L or ARL3T30N (second to the left) and complexes recovered on amylose beads were resolved by SDS-PAGE followed by Coomassie staining and immunoblotting with α-ARL3 (second to the right and right, respectively). A schematic representation of direct interactions of ARL3Q70L with BBS1 and BBS5 of the BBSome was shown (lower left). MW, molecular weight. Source data are available for this figure: SourceData F4.