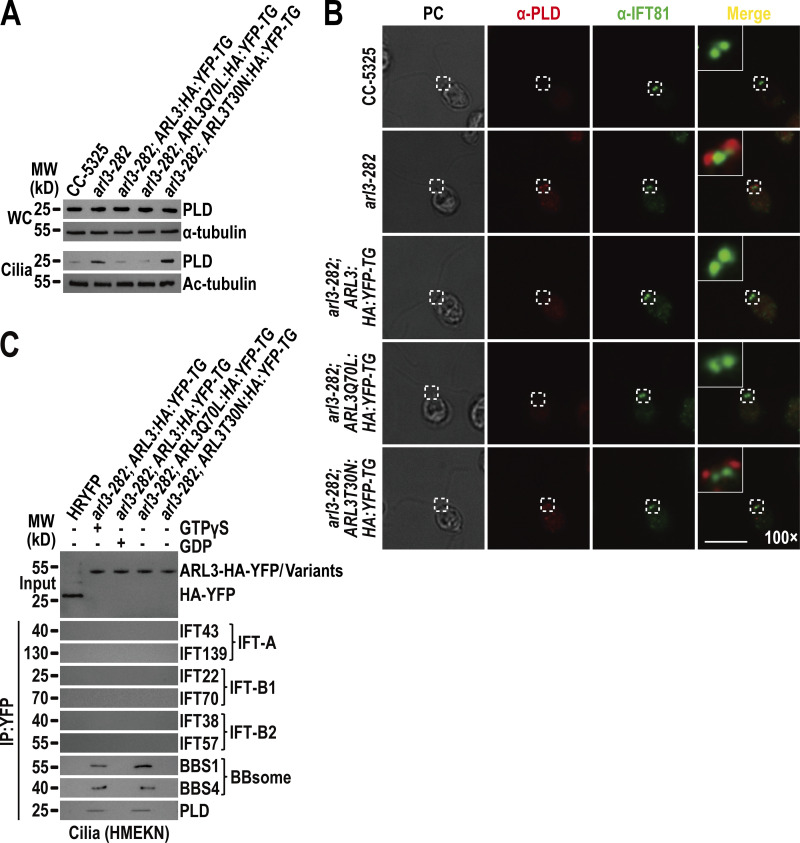
Figure 6.
ARL3GTP recruits the retrograde IFT train-shed and PLD-laden BBSome to move across the TZ for ciliary retrieval. (A) Immunoblots of WC samples and cilia of CC-5325, arl3-282, arl3-282; ARL3:HA:YFP-TG, arl3-282; ARL3Q70L:HA:YFP-TG, and arl3-282; ARL3T30N:HA:YFP-TG cells probed for PLD. α-Tubulin and acetylated-α-tubulin (Ac-tubulin) were used as a loading control for WC samples and cilia, respectively. (B) CC-5325, arl3-282, arl3-282; ARL3:HA:YFP-TG, arl3-282; ARL3Q70L:HA:YFP-TG, and arl3-282; ARL3T30N:HA:YFP-TG cells stained with α-PLD (red) and α-IFT81 (green). PC images of cells were shown. Inset shows the proximal ciliary region and the basal bodies. Inset magnification (100 times) was shown. Scale bar, 10 µm. (C) Immunoblots of α-YFP-captured proteins from HR-YFP (HA-YFP-expressing CC-125 cells), arl3-282; ARL3:HA:YFP-TG (in the presence of GTPγS or GDP), arl3-282; ARL3Q70L:HA:YFP-TG, and arl3-282; ARL3:HA:YFP-TG cilia probed for the IFT-B1 subunits IFT22 and IFT70, the IFT-B2 subunits IFT38 and IFT57, the IFT-A subunits IFT43 and IFT139, the BBSome subunits BBS1 and BBS4, and PLD. HMEKN stands for the buffer used for solving cilia. Input was quantified with α-YFP by immunoblotting. MW, molecular weight. Source data are available for this figure: SourceData F6.