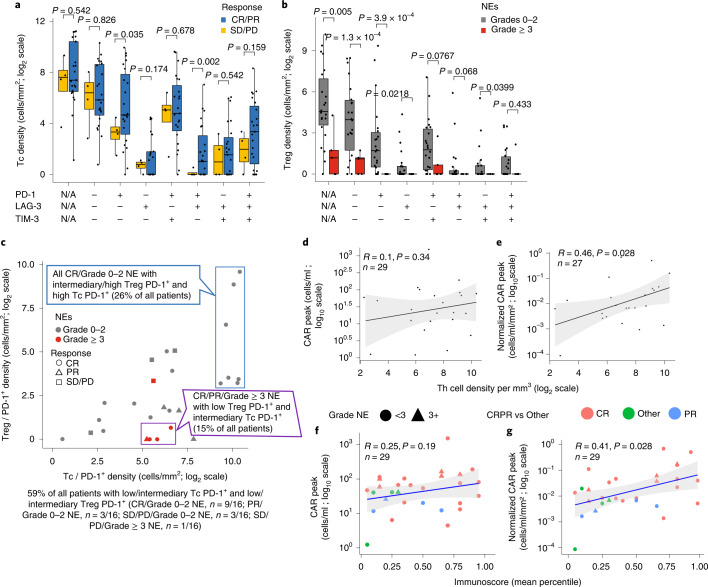
Fig. 6. Cell density of T cell subsets in pre-treatment tumor biopsies associated with axi-cel efficacy, NEs and CAR T cell expansion.
T cell subset densities were measured by Immunoscore TCE panel in ZUMA-1 tumor biopsies (n = 27) and plotted as a function of clinical response to axi-cel (CR/PR versus SD/PD; 19 CR, four PR and four SD/PD) or NE grade (grades 0–2, n = 22, versus grade ≥3, n = 5) and IC expression (PD-1, LAG-3 and TIM3). a, Tc density versus clinical response and IC expression. b, Treg density versus NE grade and IC expression. N/A = not applicable, regardless of PD-1, LAG3, TIM-3 expression (a,b). c, Correlation of clinical outcomes and NE grades with densities of tumor-infiltrating Tc and Treg cells. Two-sided exact Fisher test P value of responders with NE grade ≥3 and low Treg density (purple box) versus all other patients = 5.698 × 10−5; two-sided exact Fisher test P value of complete responders with NE grades 0–2 and high Treg density (blue box) versus all other patients = 0.02. d,e, Correlations of non-activated Th density measured by Immunoscore TCE panel and peak CAR T cells without (n = 23) (d) or with (n = 19) (e) normalization to pre-treatment TB. f,g, Correlations of Immunoscore and peak CAR T cells without (n = 29) (f) or with (n = 27) (g) normalization to pre-treatment TB. The gray ribbons (d–g) represent the 95% confidence interval of the regression line. Statistical significance of the Spearman coefficient level (two-sided P value) as shown was calculated. LAG-3, lymphocyte activation gene 3; PD, progressive disease; PD-1, programmed cell death protein 1; PR, partial response; SD, stable disease; TIM-3, T cell immunoglobulin and mucin domain 3.