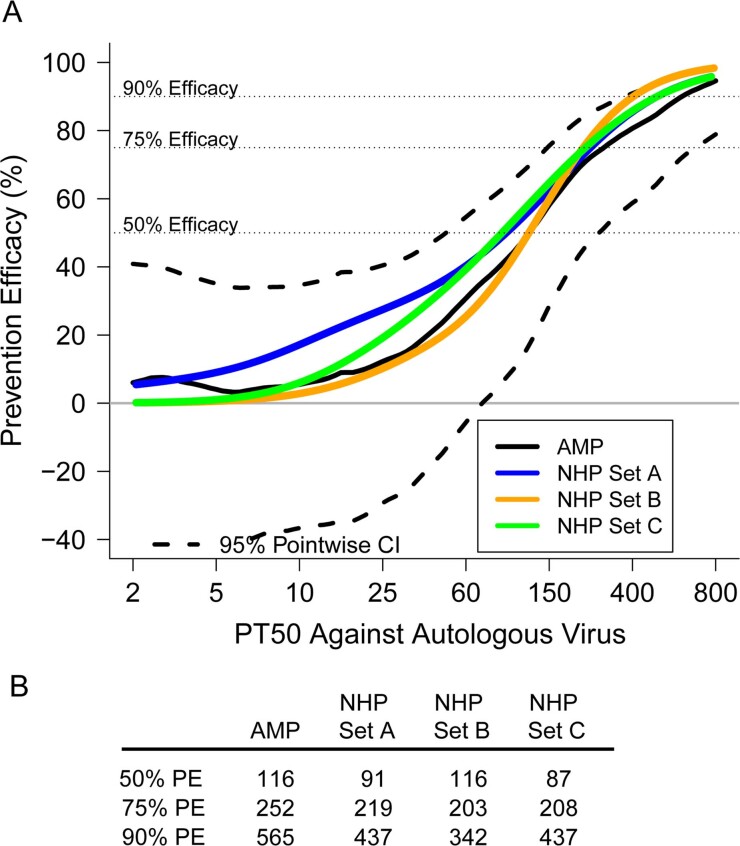
Extended Data Fig. 5. Estimated prevention efficacy (PE) by predicted serum ID50 titer (PT50) to the autologous acquired virus in the AMP trials and in non-human primate (NHP) studies.
a) Estimated PE by PT50 to the acquired virus in AMP (black line), compared to the protection curve in three different sets of NHP (blue, mustard, and green lines). b) PT50 associated with 50% PE, 75% PE, and 90% PE, for the AMP trials and for each of the three sets of NHP. Set A: N = 274 NHPs that received a single bnAb followed by SHIV challenge, bnAb titer data from all neutralization assays4; Set B: only the NHPs in Set A that received a CD4 binding site-targeting bnAb, excluding all that were challenged with SF162P3, and only including bnAb titer data from the TZM-bl target cell assay; and Set C: all NHPs in Set A, but excluding those that received an MPER-targeting bnAb and excluding all that were challenged with SF162P3, and only including bnAb titer data from the TZM-bl target cell assay.