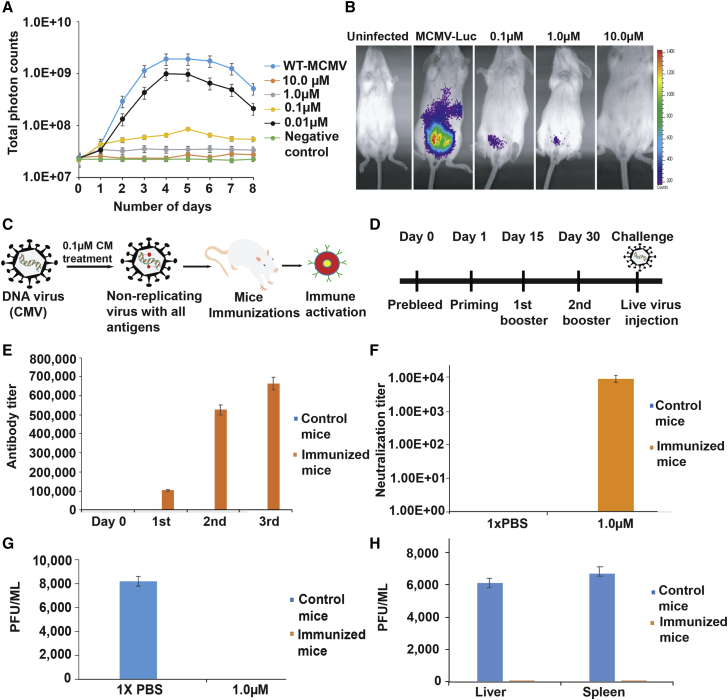
Figure 4.
CM-treated MCMV immunization provides protection in the mice
(A) Growth curve analysis of CM-treated MCMV-Luc in 3T3 cells. MCMV-Luc was treated with different concentrations of CM for 2 h at RT, followed by washing to remove unbound CM, and added to 3T3 cells in a 24-well plate at 0.1 MOI. Luciferase activity was measured as a relative light unit (RLU) using IVIS-50 on the indicated days. At 10 μM CM, MCMV-Luc was completely inactive. At 1 μM CM, MCMV-Luc can infect the cells but did not replicate. At lower CM concentrations, the virus was infectious and grew at a reduced rate. The experiment was performed in triplicate. Data are represented as mean ± SEM.
(B) In vivo analysis of CM-treated MCMV-Luc. BALB/C mice were injected with untreated and CM-treated MCMV-Luc. MCMV-Luc was treated with 0.1, 1, and 10 μM CM for 2 h at RT, followed by washing to remove unbound CM. Uninfected represents the mice without virus infection, and MCMV-Luc represents the mice injected with the virus. Mice injected with MCMV-Luc treated with 0.1, 1, and 10 μM CM are indicated. The representative images show the growth of treated and untreated MCMV-Luc in mice. MCMV-Luc was completely inactive after treatment with 10 μM CM. At 1 and 0.1 μM CM, MCMV-Luc was limited to the injected site and could not replicate. Three mice were used in each group.
(C) A pictorial representation of the DNA virus attenuation using CM and administration into the mouse. The injected mouse will produce antibodies specific to the attenuated DNA virus. The replication-defective virus carries all the antigens, infects cells, and does not form progeny virus because of alkylated DNA.
(D) A graphical representation of the timeline and strategy for mouse immunizations with CM-treated MCMV. The priming, first booster, and second booster were performed on the indicated days. Immunized mice were challenged with the live virus after 15 days from the last immunization.
(E) Antibody titers determination in the CM-treated MCMV immunized and control mice. Sera samples obtained from immunized and control mice were tested for antibody titration using ELISA. Five mice in each group were used for this assay. ELISA was performed in triplicate. p <0.05 compared with the control mice. Data are represented as mean ± SEM.
(F) An antibody neutralization assay for MCMV. Polyclonal serum samples obtained from immunized and control groups of mice were tested for neutralization titers against MCMV. CM-treated MCMV immunizations in mice generated high neutralization titers. Serially diluted serum (2-fold) samples were added to 1,000 PFUs of MCMV-Luc, followed by incubation for 1 h at RT, and added to 3T3 cells. The viral load was determined in the infected cells by IVIS. The neutralizing titer (NT50) was determined by serial dilution that inhibits a 50% reduction (half maximum) of virus entry into 3T3 cells. p <0.05 compared with control samples.
(G) Determination of viral titer in immunized mice challenged with live wild-type MCMV. Salivary glands of challenged mice were obtained after 10 days of virus administration, and tissue homogenates samples were tested for viral titer analysis using plaque assay. Immunized mice did not show any viral titers, suggesting complete protection. Five mice per group were used for this experiment. p <0.05 compared with the control mice. Data are represented as mean ± SEM.
(H) Adoptive transfer of serum from immunized mice. A passive serum transfer from immunized to unimmunized mice protects against virus challenges. Five naive BALB/C mice were injected intravenously with 150 μL pooled passive serum obtained from CM-treated MCMV immunized mice. After 24 h, the immunized animals were challenged with 10,000 PFU of MCMV. Mice injected with serum obtained from unimmunized animals were used as control. The virus titer was determined in the liver and spleen of control and immunized mice on day 7 post-challenge. Five naive mice were used in each group for this assay. p <0.05 compared with the control group of mice. Data are represented as mean ± SEM.