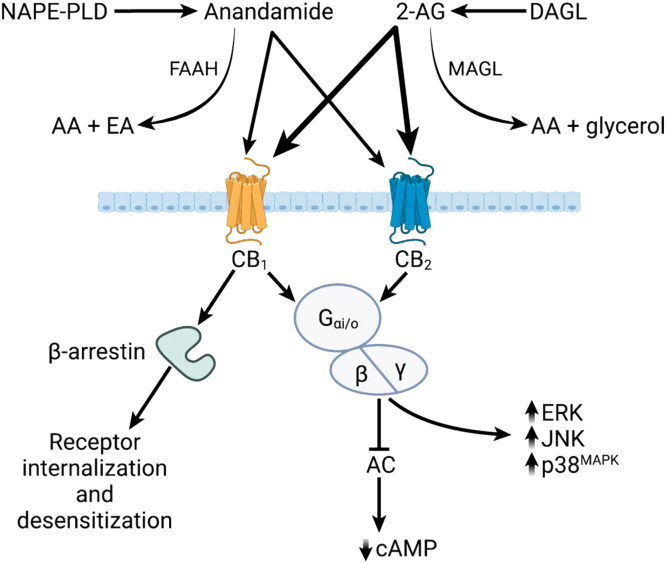
Figure 2.
Signaling pathways induced by endocannabinoid receptor activation. Anandamide is produced from N-arachidonoyl phosphatidylethanolamine (NAPE) by the action of N-acylphosphatidylethanolamine-hydrolyzing phospholipase D (NAPE-PLD). It is degraded by fatty acid amide hydrolase (FAAH). FAAH hydrolyzes AEA into arachidonic acid (AA) and ethanolamine (EA). 2-Arachidonoylglycerol (2-AG) is primarily produced through the action of diacylglycerol lipase (DAGL). It is degraded by monoacylglycerol lipase into AA and glycerol. Inhibition of these degradative enzymes increases levels of endocannabinoid to enhance signaling. Both anandamide and 2-AG signal through the CB1 and CB2 receptors where anandamide is a partial agonist (light arrows) and 2-AG is a full agonist (thick arrows). Upon ligand binding, both receptors activate Gαi/o, which then interacts with β and γ subunits to initiate downstream signaling. The primary response is inhibition of adenylate cyclase (AC) and therefore reduction in the cytosolic levels of cyclic adenosine monophosphate (cAMP). There is evidence suggesting that these receptors can also activate MAP kinase (MAPK) pathways including ERK, JNK, and p38MAPK. Activation of the CB1 receptor also triggers β-arrestin, which is involved in receptor internalization, desensitization, and degradation, and which may also be involved in intracellular signaling pathways. Figure created with BioRender.com.