Abstract
Simple Summary
Environmental productivity is considered among the key factors responsible for the uneven distribution of biodiversity on the globe, despite the lack of comprehensive studies for many groups of organisms and regions. We partly filled this gap by this study of moth diversity along the unique continent-wide gradient of environmental productivity across southern African savannahs. We revealed a significantly positive relationship of the moth species richness and environmental productivity, which we did not observe for moth abundance. We hypothesize the effects of water availability, habitat complexity, and plant diversity drive the described relationships.
Abstract
Environmental productivity, i.e., the amount of biomass produced by primary producers, belongs among the key factors for the biodiversity patterns. Although the relationship of diversity to environmental productivity differs among studied taxa, detailed data are largely missing for most groups, including insects. Here, we present a study of moth diversity patterns at local and regional scales along a continent-wide gradient of environmental productivity in southern African savannah ecosystems. We sampled diversity of moths (Lepidoptera: Heterocera) at 120 local plots along a gradient of normalized difference vegetation index (NDVI) from the Namib Desert to woodland savannahs along the Zambezi River. By standardized light trapping, we collected 12,372 specimens belonging to 487 moth species. The relationship between species richness for most analyzed moth groups and environmental productivity was significantly positively linear at the local and regional scales. The absence of a significant relationship of most moth groups’ abundance to environmental productivity did not support the role of the number of individuals in the diversity–productivity relationship for south African moths. We hypothesize the effects of water availability, habitat complexity, and plant diversity drive the observed moth diversity patterns.
Keywords: abundance, Afrotropics, Heterocera, insect, diversity patterns, light trapping, lepidoptera, NDVI, primary productivity, savannah ecosystems
1. Introduction
Environmental productivity, defined as the rate of biomass production in the ecosystem, ranks among the most studied ecological factors in relation to the global patterns of biodiversity [1,2,3,4,5]. It determines the availability of various resources which have been hypothesized to drive the intensity of interspecific competition [6] and to limit the number of coexisting species [7,8]. Additionally, the species–energy theory assumes that the habitat’s energy supply sets an upper limit to the number of individuals in a community [7], and the more individuals hypothesis (MIH) has been suggested to explain the positive linear relationship of species richness with environmental productivity [7,9]. It expects that low productivity cannot support a high number of species, since these communities would have such small populations that their extinction rates would be higher than origination rates [10]. Although several studies found that species richness has often been positively related to available energy, diversity patterns do not seem to be mediated by the number of individuals [3,11,12].
However, the relationship of animal diversity with environmental productivity (or its surrogates; see [13]) has been unevenly studied, and therefore, its general patterns remain unclear and inconsistent, especially for some groups (e.g., [1,2,5]). The existing studies and reviews revealed either linearly increasing diversity with productivity or a hump-shaped relationship with the highest diversity in the intermediately productive environments (e.g., [1,2,5]), although decreasing diversity with environmental productivity and non-significant relationships were also found (e.g., [1,2]). Some authors also suggested that the relationship varies across geographic scales (e.g., [1,2,14]). While the diversity can show the hump-shaped patterns at local scales, it increases mostly linearly with environmental productivity at regional or larger scales [14]. Nevertheless, such rules are by no means universal, as Cusens et al. [15] found no support for the scale dependency of the patterns in their meta-analysis, and the significant, positive linear relationship prevailed across the scales.
For diversity and abundance spatial patterns of insects, one of the most abundant and speciose groups in terrestrial ecosystems, environmental productivity has repeatedly been suggested as the key driver [16,17], despite the general lack of available data [9,11]. Moreover, most of the few available studies included the above-mentioned bias of the confounding effects of environmental productivity with latitude or elevation. When environmental productivity was studied independently of elevation and latitude, a significant positive linear relationship was found for butterflies [18,19], ants [20], and damselflies [21]; and marginally positive or non-significant relationships were found for butterflies [22,23] and aquatic insects [24], at both local and regional scales. In the Afrotropics, only the diversity patterns of sphingid moths were studied at larger scales, evaluating environmental productivity as one of the main responsible variables, with a strong positive correlation with species richness [25]. Nevertheless, this study relied on the diversity data from modelled species distribution, and any relationship with environmental variables could thus be artificial, as the variables had been used for the modelling as well. This highlights how poorly the drivers of insect diversity patterns were studied in the Afrotropics, particularly in the Afrotropical savannahs [26].
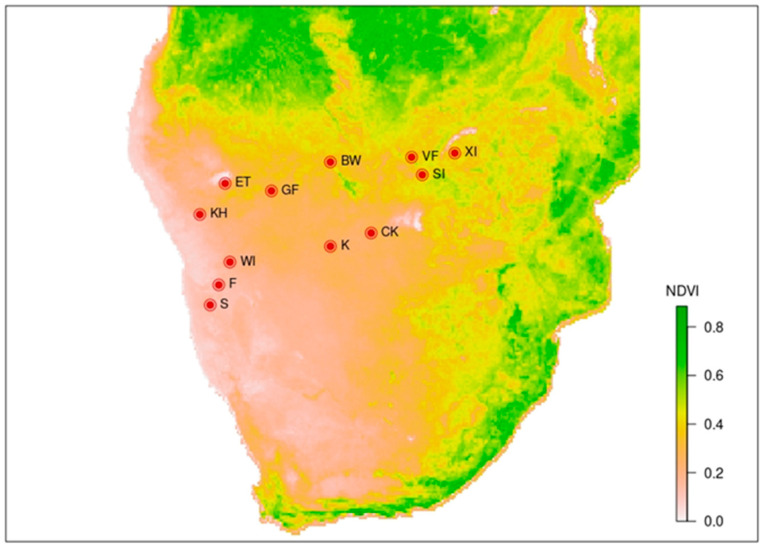
In this study, we focus on patterns of diversity and abundance of adult moths along a latitude-unrelated, continent-wide gradient of environmental productivity in southern African savannahs, at the local and regional scales. The studied gradient of environmental productivity (Figure 1) is unique in its high independency on other climate variables, especially environmental temperature, to whose gradient it is more or less perpendicular [27]. Moths are a diverse group of commonly used biodiversity indicators, with an important role in ecosystem food webs, including herbivory, prey for many predators and pollination. As mostly primary consumers, their communities can be expected to be closely related to environmental productivity. We specifically asked the following questions: (1) Do relationships between species richness and environmental productivity differ at local (alpha diversity) and regional (gamma diversity) scales? (2) How is abundance related to environmental productivity at both scales? We predicted a positive linear relationship of species richness and abundance of moths to environmental productivity at both scales. To better understand the revealed patterns, we also performed partial analyses for abundant moth subgroups.
Figure 1.
Regions in southern Africa sampled for moth communities along the gradient of environmental productivity. Mean NDVI in the beginning of vegetation season (October to December) is visualized. Region codes are listed in Table 1.
2. Materials and Methods
2.1. Data Sampling
The field sampling was conducted along a continent-wide gradient of environmental productivity in southern Africa, from the deserts in western Namibia, through semideserts and open savannahs in Namibia and Botswana, to productive woodland savannahs in northwestern Zimbabwe [28]. Along this productivity gradient, we sampled moth communities in 12 regions in open and semi-open natural habitats (Figure 1; Table 1).
Table 1.
Summary and characteristics of the regions (with their codes used in Figure 1) sampled for moth diversity along the environmental productivity gradient in southern Africa. The NDVI values and vegetation layer coverages were averaged from the 10 plots for each region.
| Region (Code) | Elevation (m a.s.l.) | Latitude/ Longitude |
Habitat Type | Max./ Mean/ Min. NDVI |
Vegetation Cover (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| All | 30 cm | 2 m | 5 m | >5 m | |||||
| Soussusvlei (S) | 760 | S 24.543° E 15.789° |
Namib Desert with very scarce vegetation | 0.1119 0.1086 0.1038 |
14.6 | 9.9 | 4.1 | 0.5 | 0.0 |
| Namibgrens (F) | 1790 | S 23.643° E 16.279° |
Namib Escarpment Woodland: dry savannahs and shrubby areas with scattered trees | 0.1448 0.1417 0.1383 |
16.8 | 6.8 | 5.3 | 4.7 | 0.0 |
| Khorixas (KH) | 1040 | S 20.440° E 15.215° |
Angolian Mopane Woodland: mosaic of Acacia and mopane woodlands | 0.1864 0.1732 0.1593 |
45.7 | 2.8 | 22.3 | 17.7 | 2.9 |
| Windhoek (WI) | 1800 | S 22.608° E 16.773° |
Namib Escarpment Woodland: dry savannahs and shrubby areas with scattered trees | 0.2276 0.1964 0.1719 |
46.9 | 26.0 | 16.2 | 4.7 | 0.0 |
| Etosha (ET) | 1120 | S 19.051° E 16.541° |
Angolian Mopane Woodland: mosaics of Acacia and mopane woodlands | 0.2837 0.2412 0.2018 |
93.7 | 39.4 | 32.7 | 21.6 | 0.0 |
| Thakadu (K) | 1120 | S 21.867° E 21.697° |
Kalahari Xeric Savannah: dry open savannahs, with scattered trees | 0.2979 0.2481 0.2047 |
106.0 | 35.5 | 49.5 | 20.5 | 0.5 |
| Central Kalahari (CK) | 980 | S 21.288° E 23.716° |
Kalahari Acacia Woodland: mosaics of Vachellia, Baikeia and mopane woodlands, and small-leaved savannahs | 0.3487 0.2586 0.1912 |
87.7 | 26.0 | 33.5 | 23.5 | 4.7 |
| Grootfontein (GF) | 1220 | S 19.346° E 18.812° |
Kalahari Acacia Woodland: mosaics of Vachellia, Baikeia and mopane woodlands, and small-leaved savannahs | 0.3554 0.2884 0.2264 |
133.2 | 55.0 | 45.0 | 27.5 | 5.7 |
| Bwabwata (BW) | 1030 | S 18.092° E 21.686° |
Zambezian Baikiaea Woodlands: mosaic of mopane and Baikeia woodlands, and secondary grasslands | 0.4459 0.3542 0.2689 |
114.6 | 39.5 | 40.3 | 12.5 | 22.3 |
| Hwange (SI) | 1020 | S 18.699° E 26.192° |
Zambezian and Mopane Woodlands: mosaic of miombo and mopane woodlands, and shrubby savannahs | 0.5468 0.4090 0.2734 |
106.1 | 42.3 | 35.5 | 18.0 | 10.3 |
| Victoria Falls (VF) | 920 | S 17.872° E 25.721° |
Zambezian and Mopane Woodlands: mosaic of mopane and Baikeia woodlands, and secondary grasslands | 0.5431 0.4222 0.3066 |
123.8 | 49.0 | 41.5 | 25.2 | 8.1 |
| Chizarira (XI) | 1010 | S 17.701° E 27.855° |
Zambezian and Mopane Woodlands: mosaic of mopane and Baikeia woodlands, and secondary grasslands | 0.5692 0.4379 0.3145 |
124.2 | 45.2 | 27.2 | 32.0 | 15.1 |
In each sampling region, 10 local plots were selected at least 1 km apart from each other, forming a 10 km transect, or two perpendicular transects in some regions. Nocturnal moths were collected using portable light traps (with 48 LED lights arranged into two strips, prevailing UV light spectrum—400 nm, 400 lm; run by a 12 V battery). The sampling was carried out during the beginning of the vegetation season, i.e., November and December (see Table S1 for particular sampling dates). The nights with forecasted temperature drop or strong wind were avoided. To decrease the effect of weather on the moth capture efficiency, sampling of individual plots in each region was split in two or three nights, whenever possible. All captured moths were euthanized by ammonium carbonate placed in a small mesh bag in each trap. A light trap was exposed for a night (from dusk till dawn) at individual local sampling plots. Moth specimens were sorted out in the field, dried by silica gel, and stored in paper envelopes. All individuals of the target moth groups (Noctuoidea: Erebidae, Eutellidae, Noctuidae, Notodontidae; Bombycoidea s.l.: Eupterotidae, Lasiocampidae, Saturniidae, Sphingidae—hereinafter referred to as Bombycoidea; Zygaenoidea: Limacodidae) were later mounted; identified by species or morphospecies through a combination of morphological characters and genitalia dissections; and counted. Specimens of Geometroidea were counted but not identified (especially because numerous specimens of tiny geometrid species would require intensive genitalia dissections which was not within our capabilities); therefore, this superfamily was used only for analyses of abundances but not for analyses of species richness. The voucher material is stored in the Biology Centre, Czech Academy of Sciences, České Budějovice, Czechia.
Environmental productivity was characterized by the normalized difference vegetation index (NDVI) for quantifying remotely sensed vegetation greenness. NDVI is a widely accepted proxy for environmental productivity, commonly applied at different spatial scales in order to predict species richness [13]. We used the NDVI values produced by an extended, 8 km Advanced Very High Resolution Radiometer (AVHRR; [29]). We used the average of monthly maximum NDVI from the beginning of the vegetation season in the studied region (i.e., from October to December) from years 1982–2004 [29]. Each local sampling plot was characterized by three measures of environmental productivity (maximum, minimum, and mean NDVI) of its 8 km grid cell. Moreover, to partly describe the habitat complexity, individual vegetation layer coverages were visually estimated at each local plot during the setting of the light traps. For the regional-scale analyses, the values of each characteristic were averaged for the 10 local plots of each region (Table 1). We tested collinearity among all described characteristics. As virtually all characteristics were intercorrelated (Pearson ρ ≥ |0.5|; Table S2), we selected mean NDVI as the only proxy for environmental productivity in our analyses.
2.2. Data Analyses
We analyzed the relationship of moth diversity with environmental productivity in R 4.0.3 [30]. All analyses were first run with the complete datasets (i.e., all moths for abundances, and all moths except Geometroidea for species richness), followed by separate analyses of particular moth groups to reveal potentially different patterns among them. Based on the numbers of sampled species and specimens (Table 2), superfamilies Bombycoidea and Noctuoidea were analyzed separately also. As families Erebidae and Noctuidae (both belonging to the Noctuoidea superfamily) were substantially abundant in our material, and they are common focal groups for diversity studies, we ran separate analyses for them as well.
Table 2.
Diversity of the focal moth groups at individual regions: gamma diversity (γ: regional species richness), alpha diversity (α: mean local species richness), abundance (Ab.: regional number of specimens).
| Region | All Moths Exc. Geometroidea | Bombycoidea | Noctuoidea | Erebidae | Noctuidae | All Moths Incl. Geometroidea | Geometroidea | ||||||||||||||
| γ | α | Ab. | γ | α | Ab. | γ | α | Ab. | γ | α | Ab. | γ | α | Ab. | Ab. | Ab. | |||||
| Soussusvlei | 10 | 1.9 | 47 | 1 | 0.1 | 1 | 9 | 1.8 | 46 | 3 | 0.8 | 23 | 6 | 1.0 | 23 | 50 | 3 | ||||
| Namibgrens | 45 | 9.7 | 1331 | 2 | 0.3 | 5 | 41 | 9.2 | 1324 | 13 | 3.1 | 57 | 28 | 6.1 | 1267 | 1532 | 201 | ||||
| Khorixas | 45 | 9.0 | 382 | 2 | 0.2 | 2 | 43 | 8.8 | 380 | 11 | 1.6 | 20 | 32 | 7.2 | 360 | 438 | 56 | ||||
| Windhoek | 32 | 7.0 | 399 | 3 | 0.4 | 4 | 28 | 6.5 | 394 | 11 | 3.2 | 195 | 17 | 3.3 | 199 | 436 | 37 | ||||
| Etosha | 39 | 9.2 | 710 | 0 | 0.0 | 0 | 39 | 9.2 | 710 | 13 | 2.8 | 50 | 25 | 5.4 | 312 | 876 | 166 | ||||
| Thakadu | 50 | 10.1 | 350 | 6 | 1.5 | 27 | 44 | 8.6 | 323 | 11 | 3.1 | 64 | 32 | 5.3 | 254 | 404 | 54 | ||||
| Central Kalahari | 74 | 18.4 | 569 | 8 | 2.5 | 54 | 66 | 15.9 | 515 | 19 | 4.3 | 69 | 47 | 11.6 | 446 | 612 | 43 | ||||
| Grootfontein | 85 | 21.3 | 1337 | 15 | 4.9 | 161 | 70 | 16.4 | 1176 | 16 | 4.4 | 696 | 50 | 10.8 | 325 | 3078 | 1741 | ||||
| Bwabwata | 92 | 23.0 | 762 | 19 | 4.8 | 149 | 71 | 17.9 | 610 | 30 | 8.0 | 399 | 39 | 9.7 | 209 | 982 | 220 | ||||
| Hwange | 145 | 36.0 | 994 | 9 | 3.3 | 124 | 125 | 27.8 | 669 | 49 | 9.6 | 249 | 73 | 17.4 | 406 | 1232 | 238 | ||||
| Victoria Falls | 179 | 44.8 | 1757 | 15 | 4.1 | 63 | 152 | 36.5 | 1574 | 71 | 16.1 | 340 | 72 | 17.6 | 1121 | 2182 | 425 | ||||
| Chizarira | 99 | 19.2 | 410 | 11 | 3.7 | 98 | 80 | 13.4 | 203 | 35 | 6.2 | 102 | 35 | 4.7 | 62 | 550 | 140 | ||||
| Region | Eutellidae | Lasiocampidae | Limacodidae | Notodontidae | Saturniidae | Sphingidae | Eupterotidae | ||||||||||||||
| γ | α | Ab. | γ | α | Ab. | γ | α | Ab. | γ | α | Ab. | γ | α | Ab. | γ | α | Ab. | γ | α | Ab. | |
| Soussusvlei | 0 | 0 | 0 | 1 | 0.1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Namibgrens | 0 | 0 | 0 | 2 | 0.3 | 5 | 2 | 0.2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Khorixas | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.1 | 1 | 1 | 0.1 | 1 | 0 | 0 | 0 |
| Windhoek | 0 | 0 | 0 | 2 | 0.2 | 2 | 1 | 0.1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.2 | 2 |
| Etosha | 1 | 1.0 | 348 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Thakadu | 0 | 0 | 0 | 2 | 0.9 | 18 | 0 | 0 | 0 | 1 | 0.2 | 5 | 1 | 0.1 | 1 | 2 | 0.3 | 4 | 1 | 0.2 | 4 |
| Central Kalahari | 0 | 0 | 0 | 4 | 1.5 | 37 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.1 | 1 | 3 | 0.9 | 16 | 0 | 0 | 0 |
| Grootfontein | 1 | 0.1 | 1 | 6 | 2.1 | 94 | 0 | 0 | 0 | 3 | 1.1 | 154 | 1 | 0.2 | 2 | 8 | 2.6 | 65 | 0 | 0 | 0 |
| Bwabwata | 1 | 0.1 | 1 | 7 | 1.5 | 47 | 2 | 0.3 | 3 | 1 | 0.1 | 1 | 4 | 1.0 | 26 | 7 | 1.9 | 67 | 1 | 0.4 | 9 |
| Hwange | 0 | 0 | 0 | 2 | 0.3 | 3 | 11 | 4.9 | 201 | 3 | 0.8 | 14 | 1 | 0.2 | 2 | 4 | 1.3 | 44 | 2 | 1.5 | 75 |
| Victoria Falls | 0 | 0 | 0 | 5 | 1.1 | 11 | 12 | 4.2 | 120 | 9 | 2.8 | 113 | 2 | 0.2 | 2 | 5 | 1.9 | 35 | 3 | 0.9 | 15 |
| Chizarira | 0 | 0 | 0 | 5 | 1.6 | 60 | 8 | 2.1 | 109 | 10 | 2.5 | 39 | 1 | 0.4 | 5 | 2 | 0.4 | 4 | 3 | 1.3 | 29 |
We tested the relationships of alpha diversity (mean number of species sampled at individual local plots in each sampling region), gamma diversity (number of species sampled at all local plots in each region), and abundance (number of all specimens at all local plots in each region) with environmental productivity (mean NDVI) by linear models (after visual checking for the normal distribution in our data). As unimodal models were found as one of the main patterns on the local scale in some other studies (see above), we also tested the relationships by unimodal models. To allow better comparison with other studies of moth communities, we also calculated Fisher’s-α diversity indices. Nevertheless, as we did not have any hypotheses for the relationship between environmental productivity and the shape of species-abundance distribution (represented by Fisher’s-α index; [31]), we did not include them in our analyses.
3. Results
In total, 12,372 individuals of the focal moth groups were captured. Among these, 9048 individuals were identified of 487 species or morphospecies (Table 2 and Table S3), and 3324 specimens of Geometroidea were counted without further identification. Fisher’s-α indices of the sampled moth communities ranged between 3.89 and 140.71 at the regional scale, and 1.19 and 23.62 at the local scale (Table S4).
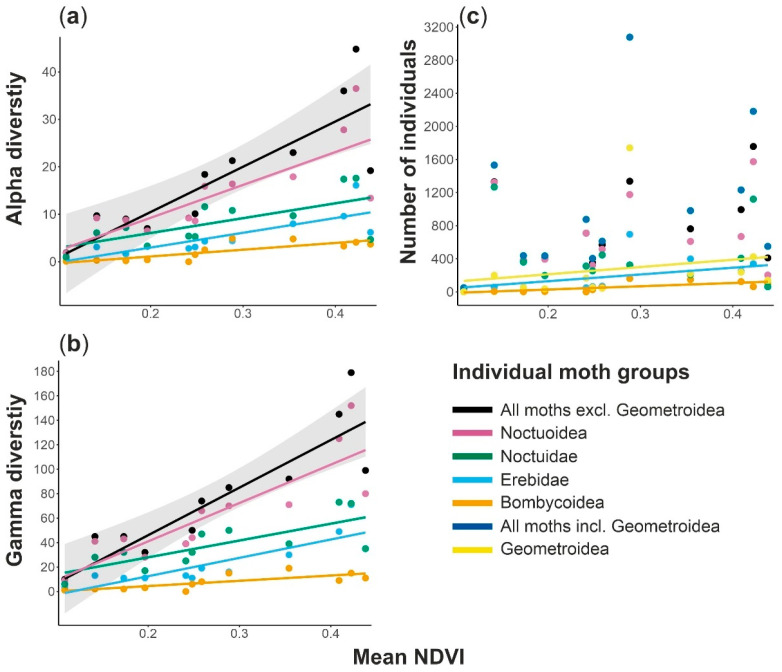
All studied groups showed significant positive linear relationships of alpha and gamma diversities with mean NDVI, whereas the unimodal relationships were non-significant for all models (Table 3, Figure 2a,b and Figure S1). For alpha diversity, the coefficients of determination R2 were greater than 60% for linear models of all moth groups but Noctuidae. All moths, excluding Geometroidea, showed higher R2 for both alpha and gamma diversity (68% and 76%, respectively) than all partial models for individual moth groups.
Table 3.
Results of linear and unimodal models for relationship of moth diversity indexes (alpha diversity, gamma diversity, and abundance) to mean NDVI for each focal moth group. Coefficients of determination (R2) are indicated, with the model p-values (n.s. p ≥ 0.05, * p < 0.05; ** p < 0.01, *** p < 0.001).
| Alpha Diversity | Gamma Diversity | Abundance | ||||
|---|---|---|---|---|---|---|
| Linear | Unimodal | Linear | Unimodal | Linear | Unimodal | |
| All moths exc. Geometroidea | 0.68 *** | 0.76 n.s. | 0.75 *** | 0.77 n.s. | 0.19 n.s. | 0.26 n.s. |
| All moths incl. Geometroidea | - | - | - | - | 0.20 n.s. | 0.29 n.s. |
| Geometroidea | - | - | - | - | 0.28 * | 0.34 n.s. |
| Bombycoidea | 0.63 ** | 0.66 n.s. | 0.51 ** | 0.47 n.s. | 0.57 ** | 0.56 n.s. |
| Noctuoidea | 0.60 ** | 0.69 n.s. | 0.71 *** | 0.73 n.s. | 0.03 n.s. | 0.15 n.s. |
| Erebidae | 0.63 ** | 0.77 n.s. | 0.82 *** | 0.80 n.s. | 0.37 * | 0.40 n.s. |
| Noctuidae | 0.39 * | 0.45 n.s. | 0.51 ** | 0.60 n.s. | −0.08 n.s. | −0.05 n.s. |
Figure 2.
Effects of environmental productivity (mean NDVI) on (a) alpha diversity (i.e., mean local species richness), (b) gamma diversity (i.e., regional species richness), and (c) numbers of individuals (i.e., abundance) in individual moth groups in southern Africa. Only the significant relationships are visualized; see Table 3 for all models results. Shaded areas indicate 95% confidence intervals for the main models with all target moth groups pooled.
Abundances of all moths, and most of the analyzed moth groups, showed non-significant relationships with mean NDVI, except Bombycoidea, Geometroidea, and Noctuidae, which showed significant positive linear relationships (Table 3, Figure 2c). Nevertheless, these correlations were relatively weak for Geometroidea and Noctuidae (R2 = 37% and 28%, respectively). The abundance of Bombycoidea correlated with mean NDVI relatively better (R2 = 57%). No significant unimodal relationships of abundance and environmental productivity were detected.
4. Discussion
The hypothesized linear increase in species richness along the environmental productivity gradient was confirmed for the local (alpha diversity) and regional (gamma diversity) scales for all analyzed moth groups. Nevertheless, a significantly positive relationship for moth abundance was found for three studied moth groups only, i.e., Geometroidea, Bombycoidea, and Erebidae, all with relatively low amounts of explained variability (Table 3). The non-significant patterns were shown for all moths, and for Noctuoidea and Noctuidae.
Our study confirmed environmental productivity as the driver of moth diversity in southern African savannahs. This finding is concordant with the results of large-scaled studies of butterfly diversity in the North American Great Basin [18,19], and of Afrotropical hawkmoths [25]. Environmental productivity also played a key role in global diversity patterns of ants [20], in the diversity of damselflies in the Amazon [21], and in the diversity of freshwater invertebrates (including insects) in ponds across 10 watersheds [14]. However, it did not significantly affect butterfly diversity across Canada, where habitat heterogeneity crucially explained the diversity and community composition of butterflies [22]. Nevertheless, the positive relationship of species richness with environmental productivity seems to prevail in insects, as supported by our results, although more data for various groups and from more areas would be needed to confirm the general pattern and to analyze its causes.
Although our results were consistent for both analyzed scales, the small-scaled relationship of local diversity and environmental productivity vary among insect taxa and regions in the available studies. Consistently with our results, environmental productivity was crucial for butterfly diversity along an elevational gradient of Mount Hernon in Israel [32]. However, on the elevational gradient of Mount Kilimanjaro, springtails and ground-dwelling beetles were the only insect groups with species richness positively related to environmental productivity [33]. Diversity of various hymenopterans showed a negative relationship, whereas species richness of moths, hoverflies, orthopterans, hemipterans, parasitoid wasps, and dung beetles had no significant relationship with environmental productivity [33]. Moreover, Chase and Leibold [14] suggested that the relationship of diversity with environmental productivity at a local scale should be expected to be hump-shaped, based on pond invertebrates, including insects. Nevertheless, such a pattern was not confirmed by any other study on insects, including our data on Afrotropical moths.
Our study did not confirm the more individual hypothesis (MIH). Due to numerous methodological constraints (e.g., [34,35]), insect abundance in communities is rarely analyzed in multi-species studies. The existing studies showed no relationship of butterfly abundance with environmental productivity across Northern America [11], and a positive relationship of ant abundance at a global scale [20]. Nevertheless, quantification of insect abundance requires more effort, especially due to the strong interannual fluctuations [35], which has not been done in any existing study, including ours. Therefore, despite the various problems of MIH [10], its validity for insects cannot be elaborately analyzed yet.
Generally, temperature was often hypothesized or even evidenced as being among the important factors influencing the diversity of ectotherms, including insects, along the environmental productivity gradients [3,5,7,9]. Nevertheless, it is often confounded with other correlates of environmental productivity because most diversity patterns have been studied along latitudinal or elevational gradients [13]. Although we have not tested this relationship, the mean temperature is known not be correlated with environmental productivity in southern Africa (and we intentionally selected our localities to be independent of environmental temperature; Figure 1), showing more complex gradients in the region [27]. Therefore, we cannot confirm that temperature can have positive effects on the diversity of south African moths. Similarly, a negative diversity–temperature relationship was documented for plants in south African savannahs [36,37].
On the other hand, precipitation, as the second of the commonly hypothesized important drivers, is positively correlated with environmental productivity in southern Africa [27,38]. Therefore, we speculate that water availability is the key component of productivity responsible for the observed southern African moth diversity patterns [39]. Insect diversity is well-known to increase in environments with complex and heterogeneous habitats [16,22,40,41,42]. We showed an increase in vegetation cover in all its layers with environmental productivity along the sampled gradient (Table S2), which supports this hypothesis.
Finally, the diversity of insects was repeatedly proven to correlate with the diversity of plants [17,42,43]. Although the relationship of plant diversity with environmental productivity may vary regionally (e.g., [44,45]), environmental productivity has recently been proven as the key driver of global plant diversity [5]. In south African savannahs, the positive diversity–productivity relationship was shown for woody plants [36], the key plant group for diversity of Afrotropical moths [42]. Altogether, we hypothesize that the observed increase in moth diversity along the environmental productivity gradient can be related to changes in water availability causing increases in diversity of plants and complexity of habitats.
Acknowledgments
We are grateful to Michal Ferenc for help in the field; Daria Ashmarina, Julie Desmist, and Inga Freiberga for preparing most of the specimens for identification; Pavel Potocký and Sara Fernández Garzón for helping with the data digitalization; Tomasz Pyrcz for providing access to the reference material in the Nature Education Centre, Jagiellonian University, Krakow, Poland; and Seth Eiseb, Iita Matheus, and Lucas Rutina for their priceless assistance with arranging permits for our research.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects13090778/s1. Table S1: Sampling dates for individual regions. Table S2: Pearson correlation coefficients among the three measures of environmental productivity, and four characteristics of vegetation cover. Table S3: Overview of the total numbers of identified species and specimens the focal moth groups. Table S4: Fisher-α diversity. Figure S1: Relationships of alpha and gamma diversities of moths with environmental productivity on log-scales.
Author Contributions
Conceptualization, D.S. and R.T.; methodology, D.S., R.T. and S.D.; formal analysis, S.D., D.S., A.T. and R.T.; investigation, S.D., R.T., V.M., O.S., D.S., T.A. and D.H.; resources, S.D., V.M., A.T. and R.T.; data curation, S.D. and R.T.; writing—original draft preparation, S.D. and R.T.; writing—review and editing, S.D., D.S., O.S., T.A., D.H., V.M., A.T. and R.T.; visualization, S.D., A.T. and R.T.; supervision, R.T. and S.D.; funding acquisition, D.S. and R.T. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This work was supported by the Czech Science Foundation (grant number 20-29554X to D.S., and grant number 18-18495S to D.S., O.S., and R.T.), and by the Charles University (PRIMUS/17/SCI/8 and UNCE204069).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Waide R.B., Willig M.R., Steiner C.F., Mittelbach G., Gough L., Dodson S.I., Juday G.P., Parmenter R. The Relationship between Productivity and Species Richness. Annu. Rev. Ecol. Syst. 1999;30:257–300. doi: 10.1146/annurev.ecolsys.30.1.257. [DOI] [Google Scholar]
- 2.Mittelbach G.G., Steiner C.F., Scheiner S.M., Gross K.L., Reynolds H.L., Waide R.B., Willig M.R., Dodson S.I., Gough L. What Is the Observed Relationship between Species Richness and Productivity? Ecology. 2001;82:2381–2396. doi: 10.1890/0012-9658(2001)082[2381:WITORB]2.0.CO;2. [DOI] [Google Scholar]
- 3.Storch D. Biodiversity and Its Energetic and Thermal Controls. In: Sibly R.M., Brown J.H., Kodric-Brown A., editors. Metabolic Ecology. Wiley; Hoboken, NJ, USA: 2012. pp. 120–131. [Google Scholar]
- 4.Pontarp M., Bunnefeld L., Cabral J.S., Etienne R.S., Fritz S.A., Gillespie R., Graham C.H., Hagen O., Hartig F., Huang S., et al. The Latitudinal Diversity Gradient: Novel Understanding through Mechanistic Eco-Evolutionary Models. Trends Ecol. Evol. 2019;34:211–223. doi: 10.1016/j.tree.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Bohdalková E., Toszogyova A., Šímová I., Storch D. Universality in Biodiversity Patterns: Variation in Species-Temperature and Species-Productivity Relationships Reveals a Prominent Role of Productivity in Diversity Gradients. Ecography. 2021;44:1366–1378. doi: 10.1111/ecog.05613. [DOI] [Google Scholar]
- 6.Grime J.P. Competitive Exclusion in Herbaceous Vegetation. Nature. 1973;242:344–347. doi: 10.1038/242344a0. [DOI] [Google Scholar]
- 7.Wright D.H. Species-Energy Theory: An Extension of Species-Area Theory. Oikos. 1983;41:496. doi: 10.2307/3544109. [DOI] [Google Scholar]
- 8.Hurlbert A.H., Stegen J.C. When Should Species Richness Be Energy Limited, and How Would We Know? Ecol. Lett. 2014;17:401–413. doi: 10.1111/ele.12240. [DOI] [PubMed] [Google Scholar]
- 9.Srivastava D.S., Lawton J.H. Why More Productive Sites Have More Species: An Experimental Test of Theory Using Tree-Hole Communities. Am. Nat. 1998;152:510–529. doi: 10.1086/286187. [DOI] [PubMed] [Google Scholar]
- 10.Storch D., Bohdalková E., Okie J. The More-Individuals Hypothesis Revisited: The Role of Community Abundance in Species Richness Regulation and the Productivity-Diversity Relationship. Ecol. Lett. 2018;21:920–937. doi: 10.1111/ele.12941. [DOI] [PubMed] [Google Scholar]
- 11.Currie D.J., Mittelbach G.G., Cornell H.V., Field R., Guegan J.-F., Hawkins B.A., Kaufman D.M., Kerr J.T., Oberdorff T., O’Brien E., et al. Predictions and Tests of Climate-Based Hypotheses of Broad-Scale Variation in Taxonomic Richness. Ecol. Lett. 2004;7:1121–1134. doi: 10.1111/j.1461-0248.2004.00671.x. [DOI] [Google Scholar]
- 12.Šímová I., Storch D., Keil P., Boyle B., Phillips O.L., Enquist B.J. Global Species-Energy Relationship in Forest Plots: Role of Abundance, Temperature and Species Climatic Tolerances: Global Species-Energy in Forest Plots. Glob. Ecol. Biogeogr. 2011;20:842–856. doi: 10.1111/j.1466-8238.2011.00650.x. [DOI] [Google Scholar]
- 13.Šímová I., Storch D. The Enigma of Terrestrial Primary Productivity: Measurements, Models, Scales and the Diversity-Productivity Relationship. Ecography. 2017;40:239–252. doi: 10.1111/ecog.02482. [DOI] [Google Scholar]
- 14.Chase J.M., Leibold M.A. Spatial Scale Dictates the Productivity—Biodiversity Relationship. Nature. 2002;416:427–430. doi: 10.1038/416427a. [DOI] [PubMed] [Google Scholar]
- 15.Cusens J., Wright S.D., McBride P.D., Gillman L.N. What Is the Form of the Productivity—Animal-Species-Richness Relationship? A Critical Review and Meta-Analysis. Ecology. 2012;93:2241–2252. doi: 10.1890/11-1861.1. [DOI] [PubMed] [Google Scholar]
- 16.Lightfoot D.C., Whitford W.G. Productivity of Creosotebush Foliage and Associated Canopy Arthropods Along a Desert Roadside. Am. Midl. Nat. 1991;125:310. doi: 10.2307/2426235. [DOI] [Google Scholar]
- 17.Wenninger E.J., Inouye R.S. Insect Community Response to Plant Diversity and Productivity in a Sagebrush–Steppe Ecosystem. J. Arid Environ. 2008;72:24–33. doi: 10.1016/j.jaridenv.2007.04.005. [DOI] [Google Scholar]
- 18.Bailey S.-A., Horner-Devine M.C., Luck G., Moore L.A., Carney K.M., Anderson S., Betrus C., Fleishman E. Primary Productivity and Species Richness: Relationships among Functional Guilds, Residency Groups and Vagility Classes at Multiple Spatial Scales. Ecography. 2004;27:207–217. doi: 10.1111/j.0906-7590.2004.03631.x. [DOI] [Google Scholar]
- 19.Seto K.C., Fleishman E., Fay J.P., Betrus C.J. Linking Spatial Patterns of Bird and Butterfly Species Richness with Landsat TM Derived NDVI. Int. J. Remote Sens. 2004;25:4309–4324. doi: 10.1080/0143116042000192358. [DOI] [Google Scholar]
- 20.Kaspari M., Ward P.S., Yuan M. Energy Gradients and the Geographic Distribution of Local Ant Diversity. Oecologia. 2004;140:407–413. doi: 10.1007/s00442-004-1607-2. [DOI] [PubMed] [Google Scholar]
- 21.Brasil L.S., Silverio D.V., Cabette H.S.R., Batista J.D., Vieira T.B., Dias-Silva K., de Oliveira-Junior J.M.B., de Carvalho F.G., Calvão L.B., Macedo M.N., et al. Net Primary Productivity and Seasonality of Temperature and Precipitation Are Predictors of the Species Richness of the Damselflies in the Amazon. Basic Appl. Ecol. 2019;35:45–53. doi: 10.1016/j.baae.2019.01.001. [DOI] [Google Scholar]
- 22.Kerr J.T., Southwood T.R.E., Cihlar J. Remotely Sensed Habitat Diversity Predicts Butterfly Species Richness and Community Similarity in Canada. Proc. Natl. Acad. Sci. USA. 2001;98:11365–11370. doi: 10.1073/pnas.201398398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hawkins B.A., Porter E.E. Water-Energy Balance and the Geographic Pattern of Species Richness of Western Palearctic Butterflies: Water-Energy Balance and Butterfly Species Richness. Ecol. Entomol. 2003;28:678–686. doi: 10.1111/j.1365-2311.2003.00551.x. [DOI] [Google Scholar]
- 24.Vinson M.R., Hawkins C.P. Broad-Scale Geographical Patterns in Local Stream Insect Genera Richness. Ecography. 2003;26:751–767. doi: 10.1111/j.0906-7590.2003.03397.x. [DOI] [Google Scholar]
- 25.Ballesteros-Mejia L., Kitching I.J., Jetz W., Nagel P., Beck J. Mapping the Biodiversity of Tropical Insects: Species Richness and Inventory Completeness of African Sphingid Moths: Mapping the Biodiversity of Tropical Insects. Glob. Ecol. Biogeogr. 2013;22:586–595. doi: 10.1111/geb.12039. [DOI] [Google Scholar]
- 26.Murphy B.P., Andersen A.N., Parr C.L. The Underestimated Biodiversity of Tropical Grassy Biomes. Phil. Trans. R. Soc. B. 2016;371:20150319. doi: 10.1098/rstb.2015.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis C.L., Vincent K. Climate Risk and Vulnerability: A Handbook for Southern Africa. Council for Scientific and Industrial Research; Stellenbosch, South Africa: 2017. [Google Scholar]
- 28.Delabye S., Sedláček O., Maicher V., Tropek R. New Records of Six Moth (Lepidoptera: Erebidae, Lasiocampidae) Species in South African Countries, with Comments on Their Distribution. Biodivers. Data J. 2020;8:e59339. doi: 10.3897/BDJ.8.e59339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tucker C.J., Pinzon J.E., Brown M.E., Slayback D.A., Pak E.W., Mahoney R., Vermote E.F., El Saleous N. An Extended AVHRR 8-km NDVI Dataset Compatible with MODIS and SPOT Vegetation NDVI Data. Int. J. Remote Sens. 2005;26:4485–4498. doi: 10.1080/01431160500168686. [DOI] [Google Scholar]
- 30.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2020. [Google Scholar]
- 31.Beck J., Schwanghart W. Comparing Measures of Species Diversity from Incomplete Inventories: An Update: Measuring Diversity from Incomplete Inventories. Methods Ecol. Evol. 2010;1:38–44. doi: 10.1111/j.2041-210X.2009.00003.x. [DOI] [Google Scholar]
- 32.Levanoni O., Levin N., Pe’er G., Turbé A., Kark S. Can We Predict Butterfly Diversity along an Elevation Gradient from Space? Ecography. 2011;34:372–383. doi: 10.1111/j.1600-0587.2010.06460.x. [DOI] [Google Scholar]
- 33.Peters M.K., Hemp A., Appelhans T., Behler C., Classen A., Detsch F., Ensslin A., Ferger S.W., Frederiksen S.B., Gebert F., et al. Predictors of Elevational Biodiversity Gradients Change from Single Taxa to the Multi-Taxa Community Level. Nat. Commun. 2016;7:13736. doi: 10.1038/ncomms13736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cardoso P., Leather S.R. Predicting a Global Insect Apocalypse: Insect Apocalypse. Insect Conserv. Divers. 2019;12:263–267. [Google Scholar]
- 35.Vagle G.L., McCain C.M. Natural Population Variability May Be Masking the More-individuals Hypothesis. Ecology. 2020;101:e03035. doi: 10.1002/ecy.3035. [DOI] [PubMed] [Google Scholar]
- 36.O’Brien E.M. Climatic Gradients in Woody Plant Species Richness: Towards an Explanation Based on an Analysis of Southern Africa’s Woody Flora. J. Biogeogr. 1993;20:181. doi: 10.2307/2845670. [DOI] [Google Scholar]
- 37.Hejda M., Čuda J., Pyšková K., Zambatis G., Foxcroft L.C., MacFadyen S., Storch D., Tropek R., Pyšek P. Water Availability, Bedrock, Disturbance by Herbivores, and Climate Determine Plant Diversity in South-African Savanna. Sci. Rep. 2022;12:338. doi: 10.1038/s41598-021-02870-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siebert A. Hydroclimate Extremes in Africa: Variability, Observations and Modeled Projections: Hydroclimate Extremes in Africa. Geogr. Compass. 2014;8:351–367. [Google Scholar]
- 39.Buckley L.B., Hurlbert A.H., Jetz W. Broad-Scale Ecological Implications of Ectothermy and Endothermy in Changing Environments: Ectothermy and Endothermy. Glob. Ecol. Biogeogr. 2012;21:873–885. [Google Scholar]
- 40.Lawton J.H. Plant Architecture and the Diversity of Phytophagous Insects. Annu. Rev. Entomol. 1983;28:23–39. doi: 10.1146/annurev.en.28.010183.000323. [DOI] [Google Scholar]
- 41.Tews J., Brose U., Grimm V., Tielbörger K., Wichmann M.C., Schwager M., Jeltsch F. Animal Species Diversity Driven by Habitat Heterogeneity/Diversity: The Importance of Keystone Structures: Animal Species Diversity Driven by Habitat Heterogeneity. J. Biogeogr. 2004;31:79–92. [Google Scholar]
- 42.Delabye S., Maicher V., Sáfián S., Doležal J., Altman J., Janeček Š., Kobe I.N., Murkwe M., Šebek P., Tropek R. Butterfly and Moth Communities Differ in Their Response to Habitat Structure in Rainforests of Mount Cameroon. Biotropica. 2021;53:567–580. [Google Scholar]
- 43.Novotny V., Drozd P., Miller S.E., Kulfan M., Janda M., Basset Y., Weiblen G.D. Why Are There So Many Species of Herbivorous Insects in Tropical Rainforests? Science. 2006;313:1115–1118. doi: 10.1126/science.1129237. [DOI] [PubMed] [Google Scholar]
- 44.Adler P.B., Seabloom E.W., Borer E.T., Hillebrand H., Hautier Y., Hector A., Harpole W.S., O’Halloran L.R., Grace J.B., Anderson T.M., et al. Productivity Is a Poor Predictor of Plant Species Richness. Science. 2011;333:1750–1753. doi: 10.1126/science.1204498. [DOI] [PubMed] [Google Scholar]
- 45.Fraser L.H., Pither J., Jentsch A., Sternberg M., Zobel M., Askarizadeh D., Bartha S., Beierkuhnlein C., Bennett J.A., Bittel A., et al. Worldwide Evidence of a Unimodal Relationship between Productivity and Plant Species Richness. Science. 2015;349:302–305. doi: 10.1126/science.aab3916. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.