Table 1.
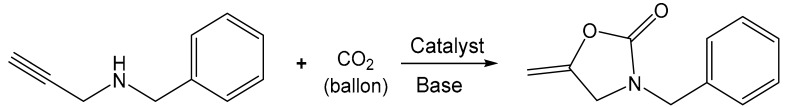
Catalysis of NPOPs and Ag@NPOPs for the carboxylative cyclization of propargylic amines with CO2 a.
| |||||||
| Entry | Catalyst | Solvent | T/°C | Base | Time/h | Yield/% | TOF/h−1 |
|---|---|---|---|---|---|---|---|
| 1 | \ | CH3CN | 50 | DBU | 2 | N.A. | |
| 2 | Ag@NPOP-1 | CH3CN | 50 | DBU | 2 | 97.0 | 1125.1 |
| 3 | Ag@NPOP-2 | CH3CN | 50 | DBU | 2 | 93.0 | 777.6 |
| 4 | NPOP-1 | CH3CN | 50 | DBU | 2 | N.A. | |
| 5 | NPOP-2 | CH3CN | 50 | DBU | 2 | N.A. | |
| 6 | Ag@NPOP-1 | CH3CN | 50 | \ | 2 | N.A. | |
| 7 | Ag@NPOP-1 | DMSO | 50 | DBU | 2 | 58 | 672.7 |
| 8 | Ag@NPOP-1 | DMF | 50 | DBU | 2 | 42 | 487.2 |
| 9 | Ag@NPOP-1 | EtOH | 50 | DBU | 2 | 15 | 174.0 |
| 10 | Ag@NPOP-1 | CH3CN | 50 | Cs2CO3 | 2 | 4.0 | 46.4 |
| 11 | Ag@NPOP-1 | CH3CN | 50 | K2CO3 | 2 | 2.0 | 23.2 |
| 12 | Ag@NPOP-1 | CH3CN | 50 | NaOH | 2 | N.A. | |
| 13 | Ag@NPOP-1 | CH3CN | 40 | DBU | 2 | 86.0 | 997.5 |
| 14 | Ag@NPOP-1 | CH3CN | 30 | DBU | 2 | 40.0 | 464.0 |
a Reaction conditions: Propargylic amine (0.2 mmol), Ag@NPOP-1 or Ag@NPOP-2 (1.0 mg), base (0.1 mmol), CO2 (balloon), solvent (1.0 mL). The reaction mixture was stirred at 50 °C for 2 h. Yield was calculated by 1H NMR with 1,4-dinitrobenzene as internal standard.
