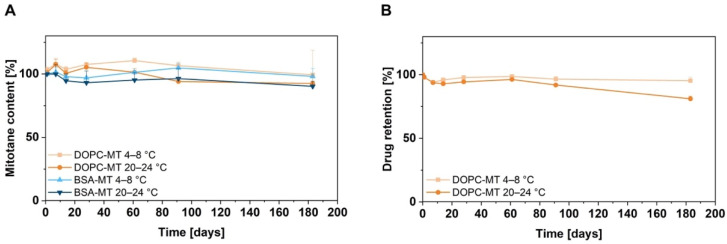
Figure 3.
Stability analysis of the mitotane content of albumin-stabilized mitotane (BSA-MT) and liposomal mitotane (DOPC-MT) under different storage conditions. Aliquots of the samples were stored at 4–8 °C or 20–24 °C for 6 months (183 days) and analyzed for their mitotane content. (A) Mitotane content given in % of the initial drug content. (B) Drug retention of DOPC-MT. A value of 100% indicates no change in drug-to-lipid ratio and thus no drug leakage from liposomes. Data are expressed as mean ± SD, n = 3.