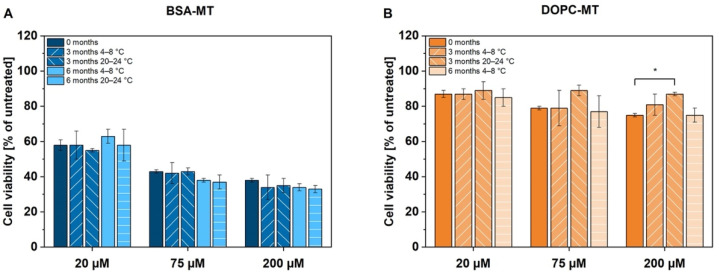
Figure 4.
Therapeutic stability analysis of (A) albumin-stabilized mitotane (BSA-MT) and (B) liposomal mitotane (DOPC-MT) under different storage conditions. Cell viability of NCI-H295R cells was analyzed by the CellTiter-Glo® luminescent cell viability assay 24 h after treatment with freshly prepared particles and particles stored for 3 and 6 months at 4–8 °C and 20–24 °C, respectively. Significant differences were evaluated via one-way ANOVA and Dunnett’s post-hoc test (* p < 0.05, further differences were not significant). Data are expressed as mean ± SD, n = 3.