Abstract
Objectives
COVID-19 has infected millions of people worldwide, with growing evidence that individuals with a history of infection may continue to show persistent post-COVID symptoms (long COVID). The aim of this study was to investigate sleep health in an international sample of individuals who reported previously testing positive for COVID-19.
Design
Cross-sectional.
Setting
Online survey distributed online between March and June 2021.
Participants
A total of 1001 individuals who reported a positive diagnosis of COVID-19 across different geographical regions, including North and South America, Sub-Saharan Africa, and Europe.
Measurements
Self-reported sleep health, using the Regulatory Satisfaction Alertness Timing Efficiency Duration scale, as recalled before a COVID-19 diagnosis and also reported currently.
Results
Individuals reported worse overall current sleep health, with lower ratings across the 6 dimensions of sleep health (sleep regularity, satisfaction, alertness, timing, efficiency, and duration) compared to their ratings as recalled before COVID-19 infection. Greater severity of COVID-19 symptoms was the strongest predictor of poor current sleep health (P < .001), independent of demographics, presence of a pre-existing chronic health condition, and time since infection. Poor current sleep health was associated with poorer current quality of life (P < .001).
Conclusions
Poor current sleep health is evident in individuals with a history of COVID-19, particularly those with more severe symptoms at the time of their COVID-19 infection and is associated with a poorer quality of life. Clinicians and researchers should assess sleep health in COVID-19 patients and investigate long-term associations with their mental and physical health, as well as potential benefits of improving sleep in this population.
Keywords: Long COVID, COVID-19, Sleep, Quality of life, General population
Introduction
COVID-19 is an infectious disease caused by the novel coronavirus SARS-CoV-2. Since the first case of COVID-19 was reported in December 2019, millions of people have been infected worldwide (WHO 2021, https://covid19.who.int/), yet the extent of its long-term consequences remains to be determined. Initially considered as a respiratory disease, COVID-19 is now recognized as a multi-organ disease,1 since the viral infection affects multiple organ systems both in the acute phase and in the long-term. COVID-19 seems to affect the nervous, autonomic, cardiovascular, respiratory/pulmonary, reproductive, immunological, and gastrointestinal systems, as well as other organ systems,2 leading to heterogeneous clinical sequelae.3
A broad range of long-term or persistent post-COVID symptoms (long COVID) has been reported in both hospitalized patients4 and in the general population,2 severely impacting quality of life.1 Especially concerning are symptoms affecting neural function, which reflect the vulnerability of the central nervous system to the novel coronavirus.5 , 6 Other coronavirus types (ie, SARS-CoV, MERS-CoV) have been shown to have the potential to infect human neural cells in the acute phase, affecting different brain areas.7 Similarly, early evidence suggests that SARS-CoV-2 may invade endothelial cells inducing brain vascular injury, thrombotic events, neurotransmitter system dysfunction, and neuronal damage—via neuroinflammation8., 9, 10.—pointing toward a possible chronic neuronal injury that might cause long-lasting neuropsychiatric symptoms.11
Sleep difficulties and fatigue along with neurocognitive dysfunction (eg, brain fog, loss of memory and attention) are common persistent symptoms apparent even several months after COVID-19 infection.2 Studies so far have shown that around 30% of individuals infected with COVID-19 experienced post-COVID sleep difficulties that persisted after the infection for up to 12 months.1 , 12., 13., 14. Frequently reported sleep difficulties that persist for several months after COVD-19 infection include newly diagnosed insomnia14 and various sleep disorders,2 , 12 including sleep apnea and restless legs syndrome.2 Sleep is likely also important for recovery in individuals with COVID-19 infection given the bidirectional relationship between sleep and the immune system.15 On the one hand, sleep is considered a critical modulator of the immune response, such that lower quality sleep can weaken the immune response, and hence, increase susceptibility to infection (viral, bacterial, or parasitic). Conversely, sleep is altered when an immune response is mounted following infection, and these alterations are believed to promote recovery during illness.15 Chronic health conditions and mental health problems are strong predictors of quality of life, and there is increasing evidence that aspects of better sleep health (eg, good sleep quality and adequate sleep duration) are associated with better quality of life.16 , 17
Sleep health is seen as a multidimensional construct,18 which considers sleep as a positive health attribute, rather than focusing on presence of a sleep complaint; this framing is more robust than unidimensional sleep assessments (eg, sleep duration) when predicting self-rated health.19 Sleep health has been associated with physical and mental health outcomes as well as quality of life19 in community populations, including in the context of the COVID-19 pandemic.20 However, to our knowledge, no data are available on the long-term effects of COVID-19 on sleep health. The aim of this study, therefore, was to investigate self-reported sleep health in an international sample of individuals who reported previously testing positive for COVID-19, considering the effects of severity of disease and time since diagnosis as well as demographic characteristics. We also investigated the association between current sleep health and quality of life. We hypothesized that greater severity of disease and shorter time since diagnosis would be related to poor current sleep health and that poor current sleep health would be associated with lower quality of life.
Method
Participants
Only individuals reporting a prior positive COVID-19 test result (through a viral or antibody test at the time of infection) participated in this study that entailed the completion of a self-reported online questionnaire. Participants were not asked to specify how many times they had been infected with COVID-19, since cases of SARS-CoV-2 reinfection were scarce at the time of data collection (March-June 2021).21 Data are presented here from 1001 individuals from 32 countries (Argentina, Bolivia, Brazil, Canada, Chile, Colombia, Comoros, Costa Rica, Dominican Republic, Ecuador, El Salvador, Germany, Guatemala, Honduras, India, Ireland, Lebanon, Malaysia, Mexico, Monaco, Panama, Paraguay, Peru, Portugal, Romania, South Africa, Spain, United Arab Emirates, United Kingdom, United States of America, Uruguay, and Venezuela), with participant age ranging between 18 and 84 years old (mean (SD), 43.5 (11. 9) years), who mostly self-identified as women (78.1%), were partnered (71.6%), and had completed at least an undergraduate degree (college/university) (71.3%). The majority were employed full-time (57%). Most respondents listed at least one pre-existing health condition before their COVID-19 infection (58.5%). Days since COVID-19 diagnosis in our sample ranged from 1 to 436. The detailed characteristics of the sample are shown in Table 1 . At the time of data collection, 413 participants (41.3%) were not treating their symptoms, 359 participants (35.9%) were receiving treatment from their medical provider, 200 participants (20%) were treating their symptoms with alternative or complimentary medicine (eg, acupuncture, herbal supplements), and 174 participants (17.4%) were using over-the-counter medicine.
Table 1.
Summary of sociodemographic characteristics of the participants (N = 1001)
| Age (years), M, SD | 43.5 | 11.9 |
|---|---|---|
| Gender (n = 1001), N, % | ||
| Man | 213 | 21.3 |
| Woman | 782 | 78.1 |
| Non-binary, transgender, or other | 6 | 0.6 |
| Country global regiona (n = 1001), N, % | ||
| East Asia and Pacific | 1 | 0.1 |
| Europe and Central Asia | 147 | 14.7 |
| Latin America and the Caribbean | 516 | 51.5 |
| North America | 218 | 21.8 |
| South Asia | 3 | 0.3 |
| Sub-Saharan Africa | 116 | 11.6 |
| Work statusb (n = 1001), N, % | ||
| Full-time employed | 571 | 57.0 |
| Part-time employed | 145 | 14.5 |
| On leave | 63 | 6.3 |
| Volunteering | 10 | 1.0 |
| Student | 48 | 4.8 |
| Unemployed | 56 | 5.6 |
| Retired | 42 | 4.2 |
| Staying at home/homemaker | 49 | 4.9 |
| Disability | 17 | 1.7 |
| Highest level of education completed (n = 1001), N, % | ||
| Some primary education (elementary school) | 5 | 0.5 |
| Completed primary education (graduated elementary school) | 5 | 0.5 |
| Some secondary education (high school) | 10 | 1.0 |
| Completed secondary education (graduated high school) | 57 | 5.7 |
| Trade/technical/vocational training | 90 | 9.0 |
| Some undergraduate education (college or university) | 121 | 12.1 |
| Completed undergraduate education | 301 | 30.1 |
| Some postgraduate education | 145 | 14.5 |
| Completed postgraduate education (masters or doctorate) | 267 | 26.7 |
| Romantic relationship status (n = 1001), N, % | ||
| Partnered | 717 | 71.6 |
| Single | 284 | 28.4 |
| Pre-existing chronic health condition (n = 1001), N, % | ||
| At least one pre-existing chronic health condition | 586 | 58.5 |
| No pre-existing chronic health condition | 415 | 41.5 |
| Days from COVID-19 diagnosis M, SD | 175.0 | 117.9 |
| Severity of symptoms while infected with COVID-19 (n = 1001), N, % | ||
| No symptoms (asymptomatic) | 35 | 3.5 |
| Some mild symptoms (no need for treatment) | 309 | 30.9 |
| Moderate symptoms (needed treatment, but no hospitalization) | 546 | 54.5 |
| Severe symptoms (hospitalization) | 84 | 8.4 |
| Critical symptoms (intensive care unit) | 27 | 2.7 |
Countries were classified into global regions according to the World Bank classification system (2017).
Work status variable was a forced-choice question.
Outcomes
Self-reported data were collected using an online survey hosted on Qualtrics (https://www.qualtrics.com/). Participants were asked to report COVID-19 test results, including the date that they had received a positive result, from which duration since COVID-19 diagnosis was calculated. Demographic information, including gender, age, romantic relationship status, educational background, work status, and country of residence, was also collected. Participants were asked about the presence of pre-existing (before COVID-19) chronic health conditions. Health conditions considered included: hypertension/high blood pressure, diabetes, chronic kidney disease, chronic liver disease (eg, cirrhosis, hepatitis B), asthma, chronic obstructive pulmonary disease, other chronic lung disease, stroke, spinal cord injury, other neurological disease (eg, Parkinson's disease, epilepsy, dementia), obesity, immunodeficiency (eg, auto-immune disease), cancer, anxiety, depression, other psychiatric disease, eating disorder, Inflammatory bowel disease/ irritable bowel syndrome, and other. We collapsed all premorbid health conditions into a dichotomous variable, “Pre-existing chronic health condition” (Yes/No). Participants also rated severity of symptoms while infected with COVID-19 (Table 1) and indicated current approaches they were using to treat their COVID symptoms.
Sleep health was assessed using the Regulatory Satisfaction Alertness Timing Efficiency Duration (RU-SATED) questionnaire,18 a standardized self-report scale that is psychometrically validated for the assessment of sleep health in adults.22 The RU-SATED scale has 6 items that assess 6 key dimensions of sleep that have been consistently associated with health outcomes: regularity (getting in and out of bed at similar times each day), subjective satisfaction (feeling satisfied with one's sleep), alertness during waking hours (ability to stay awake during the day without dozing), appropriate timing (sleeping between 2:00 AM and 4:00 AM), high efficiency (being awake for less than 30 minutes each night after trying to fall asleep), and sleep duration (obtaining between 6 and 8 hours of sleep per night). Participants were asked to rate their sleep health both before their COVID-19 diagnosis (ie, retrospectively), and currently (ie, at the time of the survey). Each item was rated on a 3-point Likert scale (0 - Rarely/Never, 1 - Sometimes, 2 - Usually/Always). To assess overall sleep health, a total score (ranging from 0 to 12) was obtained by summing all items. Higher total scores indicate better sleep health.23
Participants were also asked to rate their current quality of life (at the time of the survey) using a single-item question with responses ranging from 1 (Excellent) to 5 (Poor), with higher scores indicating worse quality of life.
Procedure
The survey took approximately 10 minutes to complete. It was developed in English by an international team of health professionals and was subsequently translated into Spanish. Both English and Spanish versions were reviewed and approved by native speakers. The survey was distributed mainly in North America, South America, Europe, and Southern Africa between March 4 and June 15, 2021, through (1) mailing lists and collaborators’ contact networks, (2) social media platforms—mainly long haulers Facebooks groups and Facebook ads, and (3) a patient database from the Center for Post-COVID Care, Mount Sinai, NY. The Mount Sinai patient database was created for research purposes. Only email addresses were accessed to allow survey links to be sent to patients.
The study was approved by the Public University of Navarra Ethical Committee (Spain, PI-003/21) and conducted in compliance with the declaration of Helsinki. Mount Sinai also provided IRB approval for access to the patient database from the Center for Post-COVID Care. Before starting the survey, participants were provided with a university ethics-committee approved informed consent specifying that participation was completely voluntary and anonymous and that they would not receive any financial compensation. Data are available from this dataset by request from the authors.
Data analysis
All descriptive statistics and analyses were conducted using IBM SPSS Statistics 27. Paired-samples t tests were used to determine whether there was a difference in current sleep health (RU-SATED total score) relative to participants’ recall of their sleep health before their COVID-19 diagnosis. Cohen's d effect sizes were calculated accounting for the longitudinal correlation between total scores, where d = 0.2 is considered a small effect size, 0.5 a medium effect size, and 0.8 a large effect size. Exploratory analyses were also conducted to determine whether the 6 dimensions of sleep health from the RU-SATED scale differed at current times (at the time of completing the survey) relative to pre-COVID, using Wilcoxon signed-rank tests. Effect sizes were analyzed using the formula rc = (abs) z/√N, where rc of 0.1 is a small effect, rc of 0.3 is a medium effect, rc of 0.5 is a large effect.
To investigate predictors of current sleep health (Total RU-SATED score, at the time of completing the survey), a hierarchical stepwise multiple linear regression was computed with current Total RU-SATED score as the outcome variable and demographic characteristics and pre-pandemic sleep health, pre-existing chronic health conditions, COVID-19 symptom severity, and days since COVID-19 diagnosis as predictors. In this regression, demographic characteristics and pre-pandemic sleep health (man vs. woman or non-binary/trans, age, education level, romantically partnered vs. not partnered, and pre-pandemic Total RU-SATED score) were included as Step 1 variables. Step 2 included whether participants reported a pre-existing chronic health condition (1 = yes, 0 = no), Step 3 included participants self-reported level of COVID-19 symptom severity while infected, and Step 4 included number of days since the COVID-19 diagnosis. Significance level was set at α < 0.05.
Next, RU-SATED total scores were compared by global region using an analysis of covariance (ANCOVA). Participants’ countries were classified by geographical world region according to the World Bank classification system. Demographics and pre-pandemic sleep health from Step 1 of the previous regression were included as covariates. Participants from South Asia, as well as East Asia and the Pacific were excluded from the ANCOVA because the small group size precluded meaningful comparisons (n = 4). Because the omnibus ANCOVA was statistically significant (noted below), 6 Bonferroni-corrected (ie, multiplying each P-value by 6) post-hoc comparisons were run to identify the specific pairwise differences in RU-SATED scores by global region.
Finally, follow-up partial correlations were run to evaluate the association between current sleep health (Total RU-SATED score) and COVID-19 severity, days since diagnosis, and current levels of quality of life, controlling for demographics and global region.
Results
Sleep health at current times relative to pre-COVID-19
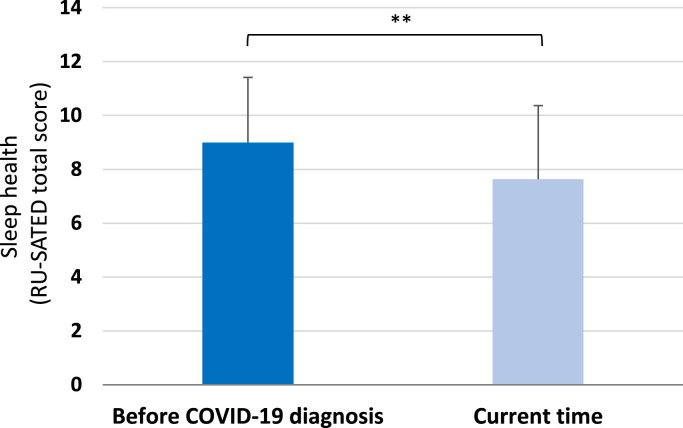
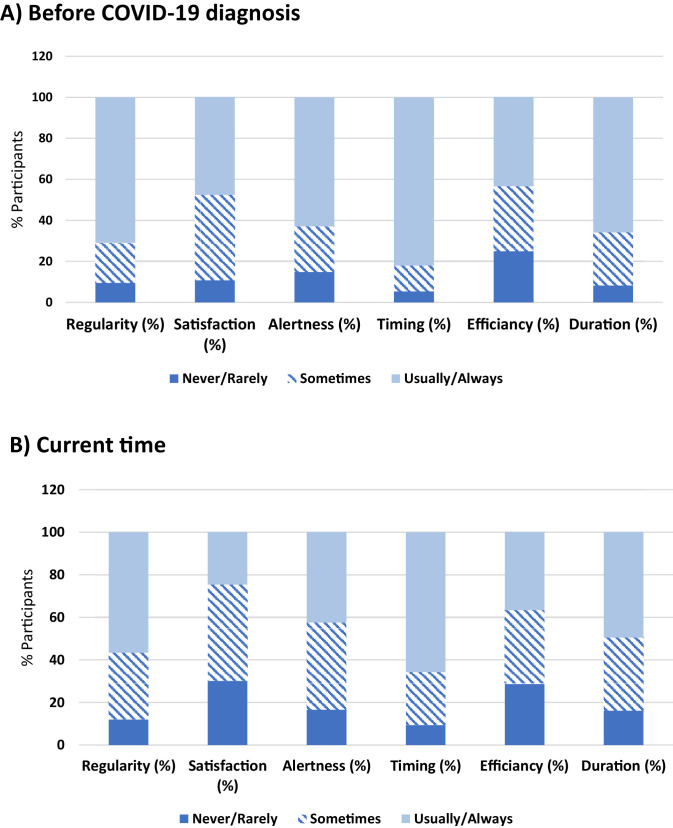
Participants had lower RU-SATED total scores (P < .001) currently (time of the survey) compared with scores as recalled before their COVID-19 diagnosis, with a medium effect size (Cohen's d = 0.52, Fig. 1 ). Participants rated all single items on the RU-SATED scale as significantly lower at the time of the survey compared with pre-COVID, with lower scores of sleep regularity (Z = -7.18, P < .001, rc = 0.23), sleep satisfaction (Z = -15.46, P < .001, rc = 0.49), daytime alertness (Z = -8.71, P < .001, rc = 0.27), sleep timing (Z = -9.98, P <.001, rc = 0.31), sleep efficiency (Z = -4.37, P < .001, rc = 0.14), and sleep duration (Z = -10.65, P < .001, rc = 0.34) (Fig. 2 ).
Fig. 1.
Participants’ sleep health (total scores in the Regulatory Satisfaction Alertness Timing Efficiency Duration (RU-SATED) questionnaire, mean (SD); n = 1001) as recalled before their COVID-19 diagnosis and reported currently. Current RU-SATED scores were significantly lower (P < .01), indicating a poorer sleep health, compared to scores as recalled before a COVID-19 diagnosis.
Fig. 2.
Percentage of participants rating the 6 dimensions of sleep health (regularity of sleep, satisfaction with sleep, alertness, timing, sleep efficiency, and sleep duration) as 0 (never/rarely), 1 (sometimes), or 2 (usually/always) as recalled before their COVID-19 diagnosis (panel A) compared with the current time (panel B). Participants rated all sleep health dimensions as lower during current times compared to before COVID-19 diagnosis (see text for details).
Prediction of current sleep health
In the hierarchical linear regression predicting current sleep health (Total RU-SATED score), Step 1 was statistically significant (F(5, 995) = 65.34, R2 = 0.247, P < .001). Sleep health was significantly and uniquely associated with being in a romantic relationship (β = 0.06, P = .030) and pre-pandemic sleep health (β = 0.49, P < .001). Sleep health was not associated with gender, age, or education. With the addition of whether participants reported a pre-existing chronic health condition as a predictor in Step 2, the overall model was still statistically significant (F(6, 994) = 55.59, R2 = 0.251, P < .001). Within this step, reporting a pre-existing chronic health condition was significantly associated with poor current sleep health (β = -0.07, P = .020). Self-reported COVID-19 symptom severity (Step 3) significantly predicted poor current sleep health (β = -0.19, P < .001), and the overall model was significant (F(7, 993) = 56.95, R2 = 0.286, P < .001). After the Step 4 addition of days since COVID-19 diagnosis, the overall model was still significant (F(8, 992) = 52.90, R2 = 0.299, P < .001,Table 2 ), and greater number of days since diagnosis was significantly associated with poor current sleep health (β = -0.11, P < .001). Within this step, being in a romantic relationship and chronic condition status were no longer significant predictors. In addition, older age now significantly predicted better sleep health in Step 4 (β = 0.06, P = .022). Notably, other than pre-pandemic sleep health, COVID-19 symptom severity emerged as the strongest predictor of sleep health out of all variables in the model at Step 4.
Table 2.
Multiple linear regression model for current sleep health (Total RU-SATED scores) with standardized B-weights from the final model that included 4 steps
| Predictor variable | β | P-value |
|---|---|---|
| Entered in step 1 | ||
| Gendera (Man = 1, Woman/Trans = 0) | 0.03 | .250 |
| Age | 0.06 | .022 |
| Education | 0.04 | .179 |
| Relationship status (1 = Partnered, 0 = Single) | 0.05 | .057 |
| Pre-pandemic sleep health | 0.46 | <.001 |
| Entered in step 2 | ||
| Pre-existing chronic health condition (1 = Yes, 0 = No) | -0.05 | .088 |
| Entered in step 3 | ||
| COVID-19 symptom severity | -0.18 | <.001 |
| Entered in step 4 | ||
| Days since COVID-19 diagnosis | -0.11 | <.001 |
RU-SATED, Regulatory Satisfaction Alertness Timing Efficiency Duration.
Gender was collapsed in a dichotomous variable to be included in the regression model.
Based on the ANCOVA predicting current sleep health, there was a statistically significant effect of global region (F(3, 988) = 13.50, P < .001, partial-eta2 = 0.039). Bonferroni-corrected post hoc pairwise comparisons showed that participants from Latin America and the Caribbean reported significantly better sleep health than participants from North America, as well as Europe and Central Asia. No other significant differences emerged.
Partial correlations, which controlled for demographic variables and global region (Table 3 ), showed that poor current sleep health was associated with a greater number of days since testing positive for COVID-19 (Spearman's ρ = -0.084, P = .008, CI: [-0.14, -0.02]) and greater symptom severity (ρ = -0.231, P < .001, CI: [-0.29, -0.17]).
Table 3.
Partial correlations adjusting for gender, age, education, romantic relationship status and global region, showed that poor sleep health (current total RU-SATED scores) was associated with a longer period of time from COVID-19 diagnosis, greater severity of COVID-19 symptoms and poorer quality of life
| ρ | P-value | |
|---|---|---|
| Days since COVID-19 diagnosis | -0.08 | .006 |
| COVID-19 symptom severity | -0.44 | <.01 |
| Quality of life (current) | -0.23 | <.01 |
RU-SATED, Regulatory Satisfaction Alertness Timing Efficiency Duration; ρ, Spearman's rho.
Current sleep health and quality of life
Current sleep health and quality of life were significantly associated with each other, controlling for demographic variables and global region (Spearman's ρ = -0.447, P < .01, CI: [-0.5, -0.4]). The relationship between current sleep health and quality of life is shown in Table 3.
Discussion
This international study aimed to investigate the multidimensional construct of sleep health and its correlates in individuals who had previously received a diagnosis of COVID-19 with a viral or antibody test. Our results indicated that participants reported worse current overall sleep health, driven by lower scores on all 6 dimensions of sleep health (sleep regularity, satisfaction, alertness, timing, efficiency, and duration), compared to the level of sleep health they recalled before being infected with COVID-19. Other than pre-pandemic sleep health, greater severity of symptoms during infection with COVID-19 (Table 1) was the strongest predictor of poor current sleep health. In contrast to our hypothesis, we found that a longer time lapse between a positive COVID-19 diagnosis and current time was independently associated with poorer current sleep health. These findings suggest that poor overall sleep health may be a persistent symptom following COVID-19 infection, and potentially, therefore, an important component of the post-acute sequalae of COVID-19, or long COVID.
To our knowledge, this is the first study investigating sleep health in association with long COVID and one of the few studies to report detailed data about sleep as self-reported following COVID-19 infection. In a single-center study of 120 patients, sleep disorders were one of the most frequently reported symptoms after an average of 110.9 days from hospital admission,12 however, the kind of sleep disorder was not defined. In a large-scale study (62,354 COVID-19 patients from 54 health care institutions in the United States) a probability of 1.9% of being newly diagnosed with insomnia in the 14-90 days after a COVID-19 diagnosis was estimated.14 In another study, prevalence of certain sleep issues (ie, insomnia, night sweats, awakened feeling unable to breath, restless legs, sleep apnea, vivid dreams, nightmares, and lucid dreams) during long COVID were examined in a survey-based study of a non-hospitalized population (3762 respondents from 56 countries).2 There was a mean prevalence of 78.6% for sleep problems, with a relatively stable probability of insomnia, sleep apnea, and other sleep problems across 7 months post-infection. A limitation of this study, however, is that the majority of participants (72%) had suspected (not confirmed) COVID-19, which makes it difficult to establish a clear relationship between reported sleep issues and viral infection.
Results from our study identified severity of symptoms of COVID-19, as the most relevant risk factor for a poor sleep health. SARS-CoV-2 causes inflammation in the brain vasculature, neurons, and supportive cells24 , 25 (see8 for a review). A sustained increase in some cerebral injury biomarkers has been found in patients with mild-to-moderate COVID-19,26 which suggests that severity of the illness and its associated immune response may play a key role in long COVID manifestation. Importantly, long COVID symptoms have been reported not only in severe or critically hospitalized patients but also in those who experienced the infection with less severe symptoms, and sometimes even in those who experienced asymptomatic infections.27 These findings support the idea that long COVID might be a direct consequence of the viral infection rather than a consequence of hospitalization and/or acute clinical interventions. In a cohort study of hospitalized patients in Wuhan (China) followed up at 6 months, sleep difficulties were one of the most common symptoms, being present in 26% of participants.13 In contrast to our findings, however, severity of COVID-19 was not associated with sleep difficulties. Different findings between studies could be due to differences in the way sleep was assessed: that study relied on a single question asking how current sleep compared with pre-COVID sleep (same, worse, better), whereas we examined the construct of sleep health involving 6 specific and essential components of sleep.
Our results also showed that having a pre-existing chronic health condition correlated with current (post-COVID infection) poor sleep health, though the effect washed out with the inclusion of additional covariates. Indeed, other studies have shown that having a pre-existing health condition, such as diabetes, obesity, or respiratory disease was a risk factor for severe symptoms of COVID-19 and hospitalization.28 , 29 It is likely that sleep health was already impaired in those individuals who had a pre-existing chronic health condition, since sleep health is associated with several health outcomes.18 , 30 Nevertheless, when considering the full sample, participants reported that their sleep health was poorer currently compared to the way they recalled their sleep health before their COVID-19 diagnosis. Sleep health, therefore, might be more compromised in individuals with a pre-existing health condition as a consequence of COVID-19 infection.
Among the demographic factors examined, being partnered, which could reflect security, support, and life stability, was associated with better sleep health, similar to prior findings in a world-wide community sample surveyed during the early stages of the pandemic.20 Older age was associated with better sleep health in the fully adjusted model. While the prevalence of sleep problems is higher in older adults, the association was significant and in the opposite direction (older age reflected better sleep health) after adjustment for comorbidities, suggesting that the presence of medical and psychiatric conditions, rather than aging itself, may be associated with poorer sleep.31 In the context of the pandemic, previous research also found that older age was associated with better sleep health in community and clinical samples, suggesting that age is protective for maintaining sleep in this context.20 , 32 , 33
Sleep is vital for overall health and emotional well-being.34 , 35 Results from our study show that individuals who have been infected with COVID-19 perceived their sleep health to be worse compared with before their infection, and that poor current sleep health is associated with lower overall quality of life. The association that we found between sleep health and quality of life in people previously infected with COVID-19 supports the literature in non-COVID populations showing that better sleep quality is associated with better quality of life.16 , 17 Others have reported that patients with long COVID have a poor quality of life4 , 12 and indeed, this may be due in part to reduced sleep health. Also, in a longitudinal study, 30.7% of COVID-19 outpatients and hospitalized patients reported worsened quality of life 6 months after diagnosis compared with baseline (at the time of acute illness).36 In the general global population, it has been reported that long COVID symptoms, especially cognitive dysfunction, have an impact on individuals’ ability to work or perform daily tasks.2 While the ongoing COVID-19 pandemic continues, the full range of clinical and economic consequences of long COVID for society remains unknown. Proposed potential contributors of infection-induced lingering symptoms, include: persistent reservoirs of SARS-CoV-2 in some tissues, COVID-19 immune dysregulation, interactions between SARS-CoV-2 and the host microbiome or virome communities, dysfunctional brainstem signaling or dysfunctional vagus nerve signaling, autoimmunity, continuing activity of primed immune cells, and problems with clotting/coagulation.3 Given that sleep and circadian timing of sleep can regulate innate and adaptive immune response, modulate viral replication within the host cells, and even determine severity of illnesses,37 more research is needed to understand the relevance of sleep in long COVID. Apart from promoting inflammatory cleansing processes and strengthening immunity,38 healthy sleep is essential to maintaining optimal brain and cognitive functioning, as well as physical and mental health.39 Conversely, sleep disturbances have a range of adverse effects on various physiological systems and induce neuroinflammation, which is known to impair cognitive function.40 Considering the complex relationships among sleep, cognition, neuroinflammation,41 and general health, sleep might not only be a symptom requiring further study, but may also be used as a recovery tool42 during long COVID. In other words, improving sleep could help improve the recovery of people suffering from long COVID and help their body, especially their immune system, to fight the negative effects of the disease. In this context, sleep health education and promotion can play an important role during post-COVID recovery to increase overall health and quality of life.
Strengths of the present study include that we used a global sample from a general population with varying levels of COVID-19 severity and duration since infection. Another strength is that we only included participants who reported having received a positive viral or antibody test for COVID-19. Finally, unlike many studies that measure only a single item or dimension of sleep quality or sleep duration, we used a multidimensional, validated measure of sleep health which allowed us to explore the effects of COVID-19 infection on multiple self-reported dimensions of sleep. There are some limitations that need to be considered as well. This study was cross-sectional, and therefore questions about pre-COVID-19 sleep and health conditions rely on recall, which might be biased by participants’ perception of the past or by memory impairment related to COVID-19 itself.26 Further, different sources used for data collection might have led to a sampling bias, and findings may not generalize to all people with COVID-19. Also, these data all relied on self-reports, and we therefore do not have clinical data about disease severity or clinical health markers or objective measures of sleep. We surveyed participants about several pre-existing medical and psychiatric conditions, which we then collapsed into a dichotomous variable and controlled for in the model. However, we did not specify obstructive sleep apnea or assess sleep apnea symptoms, which may be of higher prevalence in individuals recovering from COVID-19,2 potentially contributing to poor sleep health. The RU-SATED scale, like other broadly used sleep measures, has insufficient granularity to identify the presence of sleep disorders. Using measures of sleep such as polysomnography or actigraphy in long COVID patients would be helpful to determine whether there are changes in physiological sleep composition or objective sleep-wake measures. In chronic post-SARS individuals, non-restorative sleep with associated alpha EEG sleep anomaly and REM-related apneas/hypopneas have been reported,43 but there are no such objective sleep data in individuals with long COVID. Further, others have reported preliminary data about objective sleep quality based on actigraphy measures in 4 patients with COVID-19, however data were collected only during the sub-acute recovery stage.44 Finally, sleep health could also be affected by pandemic-related factors unrelated to contracting COVID-19, such as losing a job, financial strain, or difficulties transitioning to working from home,20 which we did not consider here. Future studies might compare sleep health in individuals diagnosed with COVID-19 and those not diagnosed from the same geographic area to establish the unique effects of long COVID on sleep.
In conclusion, poor sleep health is evident after COVID-19 infection which could have long-term consequences for mental and physical health. Future studies should investigate sleep, including objective measures of sleep and related physiology, in long COVID, as both a lingering symptom post-infection, but also as a potential tool that could be harnessed to improve overall quality of life and recovery. Sleep health promotion in the long COVID population could be beneficial, and investigations of the potential efficacy of sleep interventions for these individuals should be a priority to buffer the effects of long COVID on health and cognitive functioning.
Declaration of conflict of interest
Authors declare no conflict of interest related to the current work.
Acknowledgments
Acknowledgments
We thank all the COVID long haulers who participated in this study for their time, along with colleagues who helped by distributing the survey, especially Cristian Logatt.
Funding
Daniela Ramos-Usuga was supported by a predoctoral fellowship from the Basque Government (PRE_2019_1_0164). Dr Stella Iacovides is supported by NRF Thuthuka funding from the National Research Foundation of SA, and also NRF Incentive Funding for Rated Researchers Programme.
References
- 1.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38 doi: 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Proal AD, VanElzakker MB. Long COVID or Post-acute Sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.698169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meinhardt J, Radke J, Dittmayer C, et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci. 2021;24(2):168–175. doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- 6.Montalvan V, Lee J, Bueso T, De Toledo J, Rivas K. Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin Neurol Neurosurg. 2020;194 doi: 10.1016/j.clineuro.2020.105921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamashita M, Yamate M, Li GM, Ikuta K. Susceptibility of human and rat neural cell lines to infection by SARS-coronavirus. Biochem Biophys Res Commun. 2005;334(1):79–85. doi: 10.1016/j.bbrc.2005.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boldrini M, Canoll PD, Klein RS. How COVID-19 affects the brain. JAMA Psychiatry. 2021;78(6):682–683. doi: 10.1001/jamapsychiatry.2021.0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phetsouphanh C, Darley D, Howe A, et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nature immunology. 2022;23(2):210–216. doi: 10.1038/s41590-021-01113-x. [DOI] [PubMed] [Google Scholar]
- 10.Stefano GB, Büttiker P, Weissenberger S, Martin A, Ptacek R, Kream RM. Editorial: the pathogenesis of long-term neuropsychiatric COVID-19 and the role of microglia, mitochondria, and persistent neuroinflammation: a hypothesis. Med Sci Monit. 2021;27 doi: 10.12659/MSM.933015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng Q, Yang Y, Gao J. Infectivity of human coronavirus in the brain. EBioMedicine. 2020;56 doi: 10.1016/j.ebiom.2020.102799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garrigues E, Janvier P, Kherabi Y, et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. 2020;81(6):e4–e6. doi: 10.1016/j.jinf.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taquet M, Luciano S, Geddes JR, Harrison PJ. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry. 2021;8(2):130–140. doi: 10.1016/S2215-0366(20)30462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Besedovsky L, Lange T, Haack M. The sleep-immune crosstalk in health and disease. Physiol Rev. 2019;99(3):1325–1380. doi: 10.1152/physrev.00010.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faubel R, Lopez-Garcia E, Guallar-Castillon P, et al. Sleep duration and health-related quality of life among older adults: a population-based cohort in Spain. Sleep. 2009;32(8):1059–1068. [PMC free article] [PubMed] [Google Scholar]
- 17.Magee CA, Caputi P, Iverson DC. Relationships between self-rated health, quality of life and sleep duration in middle aged and elderly Australians. Sleep Med. 2011;12(4):346–350. doi: 10.1016/j.sleep.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 18.Buysse DJ. Sleep health: can we define it? Does it matter? Sleep. 2014;37(1):9–17. doi: 10.5665/sleep.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dalmases M, Benitez ID, Mas A, et al. Assessing sleep health in a European population: results of the Catalan Health Survey 2015. PLoS One. 2018;13(4) doi: 10.1371/journal.pone.0194495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuksel D, McKee GB, Perrin PB, et al. Sleeping when the world locks down: correlates of sleep health during the COVID-19 pandemic across 59 countries. Sleep Health. 2021;7(2):134–142. doi: 10.1016/j.sleh.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Kaperak C, Sato T, Sakuraba A. COVID-19 reinfection: a rapid systematic review of case reports and case series. J Investig Med. 2021;69(6):1253–1255. doi: 10.1136/jim-2021-001853. [DOI] [PubMed] [Google Scholar]
- 22.Ravyts SG, Dzierzewski JM, Perez E, Donovan EK, Dautovich ND. Sleep health as measured by RU SATED: a psychometric evaluation. Behav Sleep Med. 2021;19(1):48–56. doi: 10.1080/15402002.2019.1701474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalmases M, Benítez I, Sapiña-Beltran E, et al. Impact of sleep health on self-perceived health status. Sci Rep. 2019;9(1):7284. doi: 10.1038/s41598-019-43873-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanberg N, Ashton NJ, Andersson LM, et al. Neurochemical evidence of astrocytic and neuronal injury commonly found in COVID-19. Neurology. 2020;95(12):e1754–e1759. doi: 10.1212/WNL.0000000000010111. [DOI] [PubMed] [Google Scholar]
- 25.Reichard RR, Kashani KB, Boire NA, Constantopoulos E, Guo Y, Lucchinetti CF. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020;140(1):1–6. doi: 10.1007/s00401-020-02166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ameres M, Brandstetter S, Toncheva AA, et al. Association of neuronal injury blood marker neurofilament light chain with mild-to-moderate COVID-19. J Neurol. 2020;267(12):3476–3478. doi: 10.1007/s00415-020-10050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11(1):16144. doi: 10.1038/s41598-021-95565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi Q, Zhang X, Jiang F, et al. Clinical characteristics and risk factors for mortality of COVID-19 patients with diabetes in Wuhan, China: a two-center, retrospective study. Diabetes Care. 2020;43(7):1382–1391. doi: 10.2337/dc20-0598. [DOI] [PubMed] [Google Scholar]
- 29.Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28(7):1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cedernaes J, Schiöth HB, Benedict C. Determinants of shortened, disrupted, and mistimed sleep and associated metabolic health consequences in healthy humans. Diabetes. 2015;64(4):1073–1080. doi: 10.2337/db14-1475. [DOI] [PubMed] [Google Scholar]
- 31.Ancoli-Israel S. Sleep and its disorders in aging populations. Sleep Med. 2009;10(suppl 1):S7–11. doi: 10.1016/j.sleep.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Pinto J, van Zeller M, Amorim P, et al. Sleep quality in times of COVID-19 pandemic. Sleep Med. 2020;74:81–85. doi: 10.1016/j.sleep.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carrigan N, Wearn A, Meky S, et al. Sleep quality, mental health and circadian rhythms during COVID lockdown – results from the SleepQuest Study. MedRxiv preprint. 2020 [Google Scholar]
- 34.Chow CM. Sleep and wellbeing, now and in the future. Int J Environ Res Public Health. 2020;17(8):2883. doi: 10.3390/ijerph17082883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haack M, Mullington JM. Sustained sleep restriction reduces emotional and physical well-being. Pain. 2005;119(1-3):56–64. doi: 10.1016/j.pain.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 36.Logue JK, Franko NM, McCulloch DJ, et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open. 2021;4(2) doi: 10.1001/jamanetworkopen.2021.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhuang X, Rambhatla SB, Lai AG, McKeating JA. Interplay between circadian clock and viral infection. J Mol Med (Berl) 2017;95(12):1283–1289. doi: 10.1007/s00109-017-1592-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zielinski MR, Krueger JM. Sleep and innate immunity. Front Biosci (Schol Ed) 2011;3:632–642. doi: 10.2741/s176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eugene AR, Masiak J. The neuroprotective aspects of sleep. MEDtube Sci. 2015;3(1):35–40. [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu B, Dong Y, Xu Z, et al. Sleep disturbance induces neuroinflammation and impairment of learning and memory. Neurobiol Dis. 2012;48(3):348–355. doi: 10.1016/j.nbd.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clark IA, Vissel B. Inflammation-sleep interface in brain disease: TNF, insulin, orexin. J Neuroinflammation. 2014;11:51. doi: 10.1186/1742-2094-11-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Irwin MR. Sleep and inflammation: partners in sickness and in health. Nat Rev Immunol. 2019;19(11):702–715. doi: 10.1038/s41577-019-0190-z. [DOI] [PubMed] [Google Scholar]
- 43.Moldofsky H, Patcai J. Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post-SARS syndrome; a case-controlled study. BMC Neurol. 2011;11:37. doi: 10.1186/1471-2377-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vitale JA, Perazzo P, Silingardi M, Biffi M, Banfi G, Negrini F. Is disruption of sleep quality a consequence of severe COVID-19 infection? A case-series examination. Chronobiol Int. 2020;37(7):1110–1114. doi: 10.1080/07420528.2020.1775241. [DOI] [PubMed] [Google Scholar]