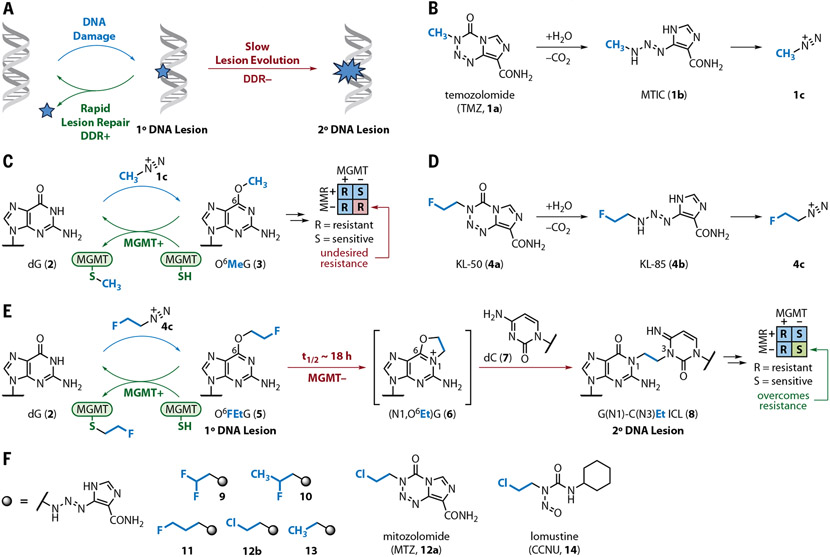
Fig. 1. Overview of mechanistic strategy and structures of agents used in this study.
(A) Underlying mechanistic design. Systemic administration of a bifunctional agent is envisioned to form a primary lesion that is rapidly resolved by healthy (DDR+) but not DDR-deficient (DDR−) cells. The persistence of the primary lesion allows it to evolve slowly to a more toxic secondary lesion. (B) Under physiological conditions, TMZ (1a) converts to MTIC (1b), which in turn decomposes to methyl diazonium (1c).(C) O6-Methylguanine (3) is the most clinical significant alkyl nucleobase generated by methyl diazonium (1c) and is rapidly reverted to dG (2) by MGMT. (D) Conversion of KL-50 (4a) to KL-85 (4b) in aqueous solution followed by decomposition to 2-fluoroethyl diazonium ion (4c).(E) 2-fluoroethyl diazonium ion (4c), derived from KL-50 (4a), is proposed to lead to the formation of G(N1)–C(N3)Et ICL 8 specifically in MGMT− cells. This mechanism is independent of MMR status. (F) Structures of the triazenes 9 to 13, mitozolomide 12a, and lomustine (CCNU; 14). Syntheses are available in the supplementary materials.