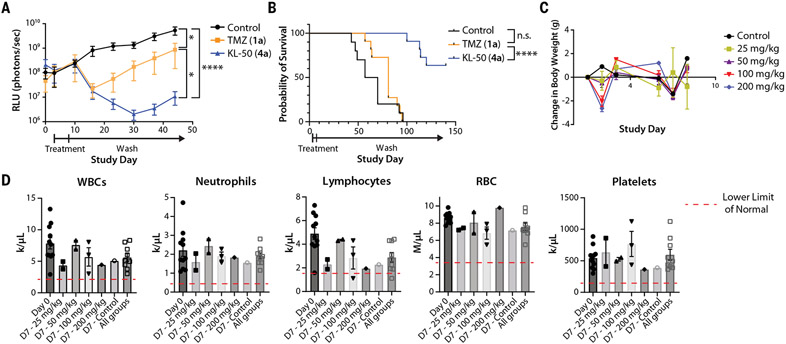
Fig. 6. KL-50 (4a) is efficacious in an LN229 MGMT−/MMR− intracranial model and is well tolerated with limited myelosuppression at supratherapeutic doses.
(A) Mean tumor size as measured with bioluminescent imaging as relative light units (RLU; photons/s) with SEM of xenograft LN229 MGMT−/MMR− intracranial tumors treated with 5 consecutive days of oral administration of 10% cyclodextrin control (n = 10 mice), TMZ (1a) (n = 11 mice, 25 mg/kg), or KL-50 (4a) (n = 11 mice, 25 mg/kg) (individual spider plots are provided in fig. S10D). (B) Kaplan-Meier analysis of intracranial xenograft tumor-bearing mice in (A), treated with 5 consecutive days of oral administration of 10% cyclodextrin control (n = 10 mice), TMZ (1a) (n = 11 mice, 25 mg/kg), or KL-50 (4a) (n = 11 mice, 25 mg/kg) demonstrating a significant survival benefit for KL-50 (4a) compared with both control and TMZ (1a) groups. (C) Mean body weight change with SEM of mice during maximum tolerated dose experiment in non-tumor-bearing mice. (D) Complete blood counts for mice before treatment and 7 days after treatment with escalations of single-dose KL-50 (4a) delivered orally. White blood cells (WBC) lower limit of normal (LLN), 2.2 k/μL; neutrophil LLN, 0.42 k/μl; lymphocyte LLN, 1.7 k/μl; red blood cells (RBC) LLN, 3.47 M/μl; platelet LLN, 155 k/μl. *P < 0.05; ****P < 0.0001; n.s.; not significant.