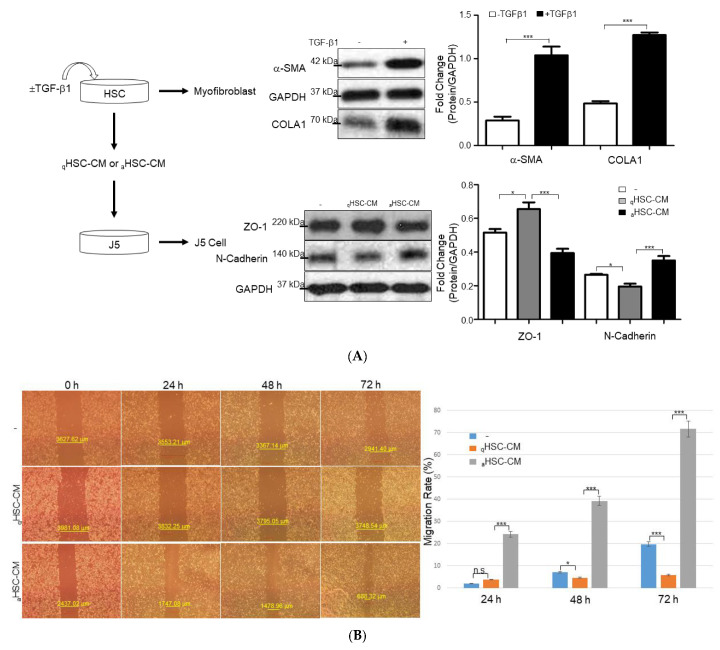
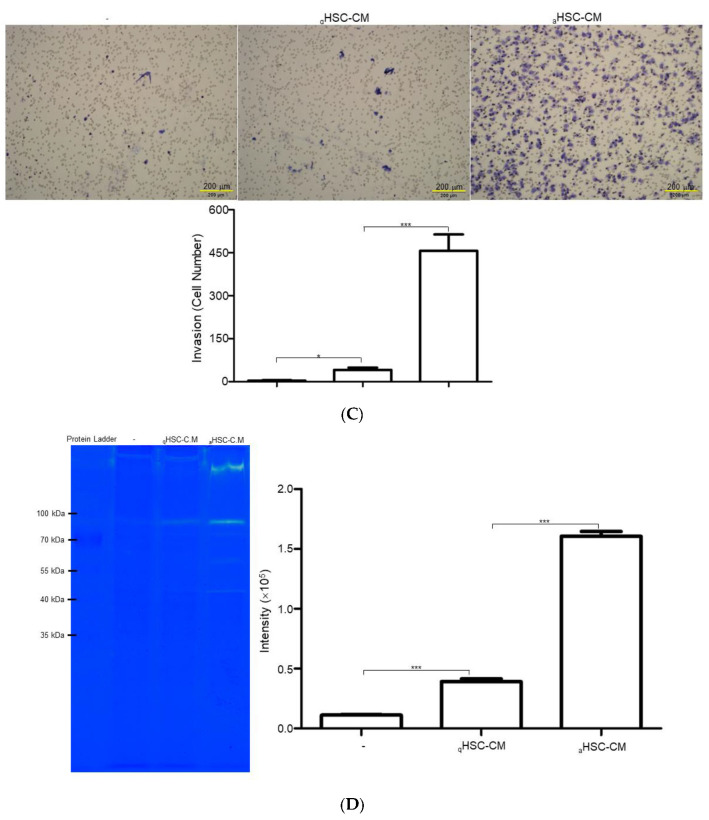
Figure 2.
The efficacy of HSC-CM upon HCC J5 cells. (A) Validation of the changes of protein levels in HSCs treated with or without TGF-β1 and J5 cells exposed to aHSC-CM or qHSC-CM through Western blot analysis. GAPDH was used as the loading control. The quantified results were indicated by the bar chart (* p < 0.05, *** p < 0.001). (B) aHSC-CM administration enhanced wound closure. Representative phase-contrast micrographs of scratch-wounded confluent cultures with regular DMEM (−), qHSC-CM, and aHSC-CM-treated J5 cells at 0, 24, 48, and 72 h post-wounding (* p < 0.05, *** p < 0.001, n.s. indicated no significance). (C) A marked increase in cell invasion was observed in the human liver cancer J5 cells treated with aHSC-CM, compared to that of regular DMEM (−) and qHSC-CM (bar scale 200 μm). A comparison of the number of transmembrane cells indicates 88% enhancement after the administration of aHSC-CM. The quantified results were demonstrated by the bar chart and represent the mean ± SD of three independent experiments (* p < 0.05, *** p < 0.001). (D) Gelatin zymographic assay was performed using the medium of J5 cells treated with regular DMEM (−), qHSC-CM, and aHSC-CM. The quantitative results were shown as bar charts (*** p < 0.001).