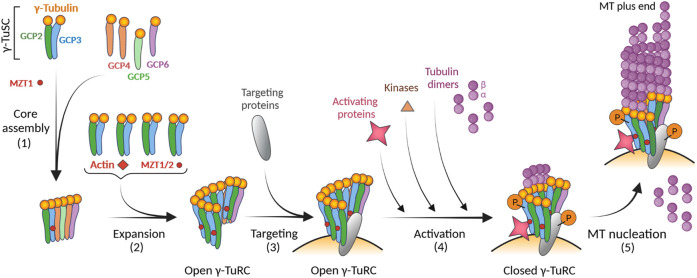
FIGURE 3.
Model for the modular assembly of γ-TuRC and its activation. The first step in the formation of γ-TuRC is the core assembly of the stable subcomplex from γ-TuSC (2 molecules of γ-tubulin and one copy each of GCP2 and GCP3), GCP-γ-tubulin heterodimers (spokes; one molecule of γ-tubulin and one copy each of GCP4, GCP5 or GCP6), and MZT1 (1). During the expansion phase, four additional γ-TuSC units, MZT1/2, and actin are added (2). The resulting γ-TuRCs with the open conformation are concentrated onto centrosomes via targeting proteins (e.g., CDK5RAP2, NEDD1) (3). The pitch and diameter of open γ-TuRC are incompatible with those of assembled microtubules. This suggests that the complex undergoes a conformational change through its activation to reduce its diameter before microtubule nucleation. Different modes of activation, including direct binding of activating proteins (e.g., CDK5RAP2, NME7), phosphorylation of γ-TuRC by kinases, or increased concentration of αβ-tubulins, can result in a conformational change leading to a closed γ-TuRC (4). Different types of activation may occur simultaneously. Nucleation-competent γ-TuRC with a closed conformation can then effectively nucleate microtubule (MT) (5). Created with BioRender.com.