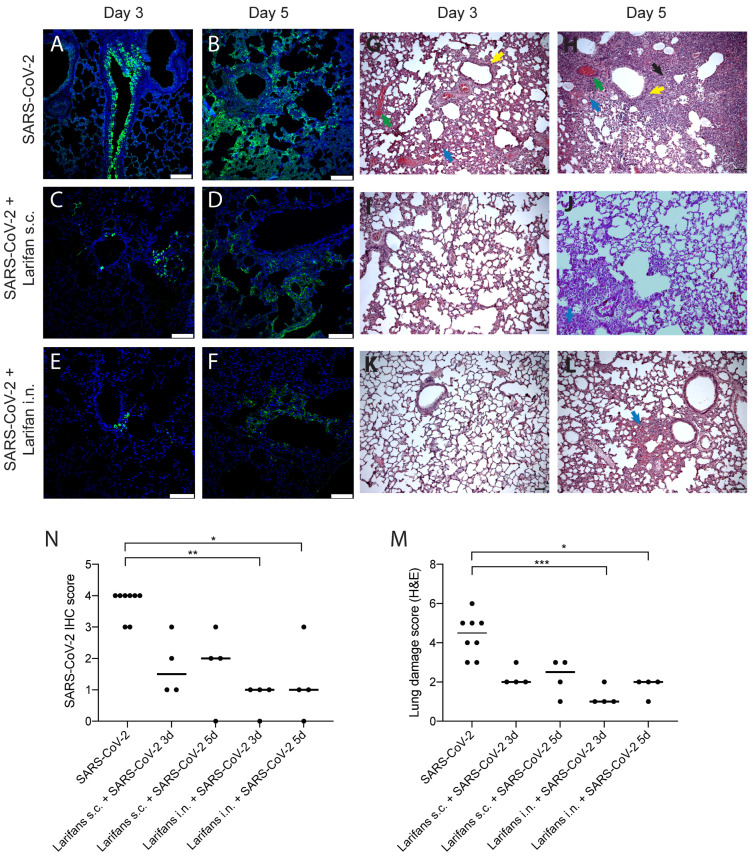
Figure 4.
Larifan treatment reduces SARS-CoV-2 antigens in the lungs and improves lung histopathological features of infected animals. Representative immunohistopathology for SARS-CoV-2 NP (green) (A–F) and hematoxylin- and eosin-stained (G–L) images of lungs of infected and infected Larifan-treated hamsters. (A) Lungs of infected untreated hamster three days post-infection; immunoreactivity predominantly located in the bronchiolar epithelium. (B) Lungs of infected untreated hamster five days post-infection; immunoreactivity observed in different alveolar regions with patchy distribution pattern. (C,D) Lungs of infected and treated hamster three and five days after Larifan s.c. administration. (E,F) Lungs of infected and treated hamster three and five days after Larifan i.n. administration. (G,H) Lungs of infected untreated hamster three and five days post-infection: thickening of the interalveolar septa due to inflammatory infiltrate (blue arrow), vascular thrombosis (red arrow), alveolar septum fibrosis (black arrow), and signs of the bronchial epithelium hyperplasia (yellow arrow). (I,J) Lungs of infected and treated hamster three and five days after Larifan s.c. administration. (K,L) Lungs of infected and treated hamster three and five after Larifan i.n. administration. (M) The score of immunolabeled virus NP in lungs of infected untreated and infected Larifan-treated hamsters. (N) Histopathological damage severity score of hamster lungs of infected untreated and infected Larifan-treated animals. Data presented as mean. Statistical significance analysis was performed using Kruskal–Wallis tests with Dunn’s multiple comparison test. * p < 0.05, ** p < 0.01, *** p < 0.001. Dots represent individual hamsters. Scale bars 100 µm.