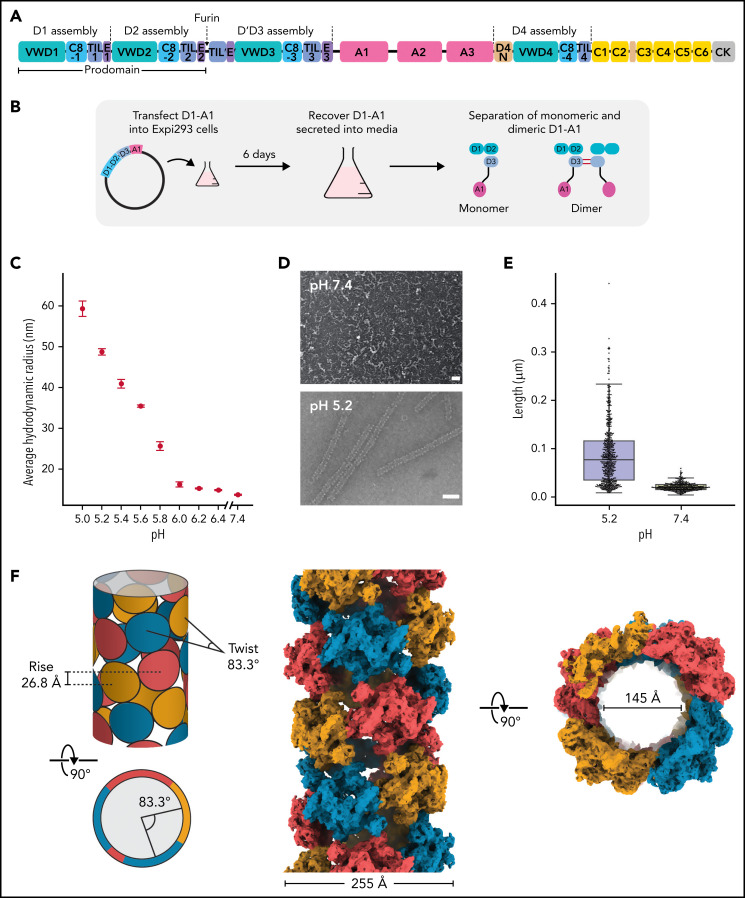
Figure 1.
Structure of a VWF tubule. (A) Schematic of the domain organization of the VWF proprotein. Each D assembly except D′ is made of domains VWF type D domain, C8, TIL (trypsin inhibitor-like cysteine-rich domain), and E. D1 and D2 form a prodomain that is cleaved by furin protease before secretion. (B) Strategy to obtain VWF monomers and disulfide-linked dimers. VWF D1-A1 was expressed in Expi293 cells and purified from the media as monomers and disulfide-linked dimers. The furin cleavage site was mutated to ASAS to prevent prodomain dissociation. (C) DLS experiments of purified D1-A1 incubated at indicated pH showing an increase in average hydrodynamic radius with a decrease in pH. Error bars represent the difference between the means from 2 replicates. (D) Representative micrographs from negative-stain electron microscopy showing VWF dimers incubated at pH 7.4 and 5.2 (scale bars, 50 nm). (E) Quantification of (D) showing particle lengths measured from 3 micrographs from samples incubated together overnight. The bottom, middle, and top lines of the shaded boxes represent the first quartile, the median, and the third quartile of measured particle length, respectively. The length of the whiskers below and above the box plot represent the lesser of the range of data or 1.5 multiplied by the interquartile range. (F) Schematic and cryo-EM structure of the dimer-derived tubule showing the bead-like arrangement. The structure forms a right-handed helix with a helical rise of 26.8 Å and a helical twist of 83.3°. The cryo-EM map has been Gaussian filtered for visualization.