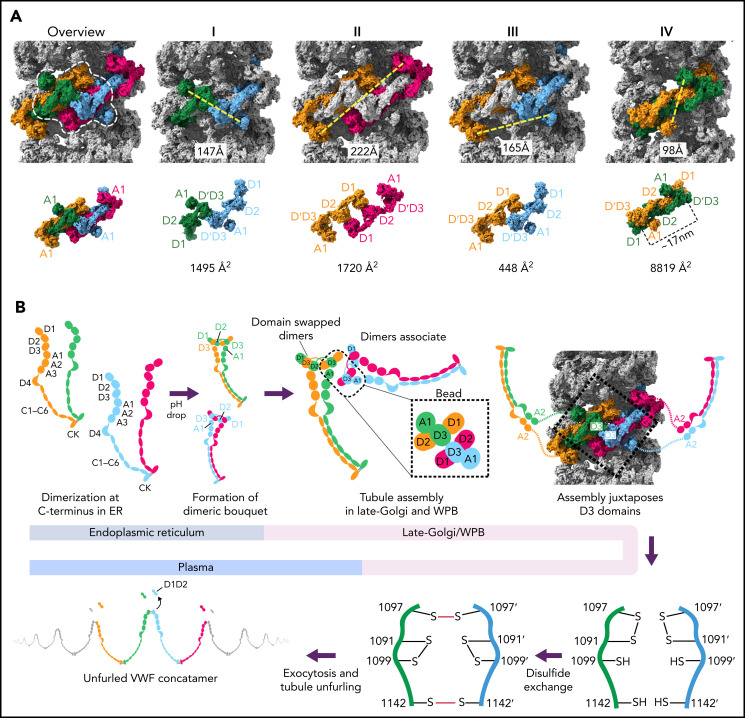
Figure 7.
Model for VWF tubule assembly. (A) Options I-IV show the possible molecules that could be disulfide bonded at their C-termini in native tubules generated with full-length VWF. Distances between A1 domains (calculated between A1464 Cα positions) are shown below, together with surface representations of potential C-terminally–linked dimers and their quantified interfacial surface area. An overview is provided with a single bead denoted with a dashed white outline. Interfacial surface areas were calculated using PDBePISA.41 (B) Schematic showing a hypothetical maturation pathway for full-length VWF based on our cryo-EM structures and prior work. In this model, the acidic environment of the Golgi induces 2 changes in the VWF dimer: the A2-CK domains zipper together, and the N-terminal heads form a “dimer swapped” conformation with D3 of one monomer nestling in a D1-D2 cradle of the other monomer. N-terminal dimer association leads to tubule assembly. Within the tubule, D3 domains from different dimers are juxtaposed. Once positioned, a disulfide exchange occurs where C1097 is liberated from an intramolecular disulfide bond to form 1 of 2 disulfides that concatemerize VWF dimers at their D3 domain. The second disulfide, between adjacent C1142 residues, occurs without disulfide exchange. With dimers linked at their D3 domains, the VWF tubule can unfurl into a high molecular weight VWF concatemer upon exocytosis into the blood plasma.