Fig. 5. Low concentrations of VRC07 IgG1 protect against repetitive HIV vaginal challenge in BLT humanized mice.
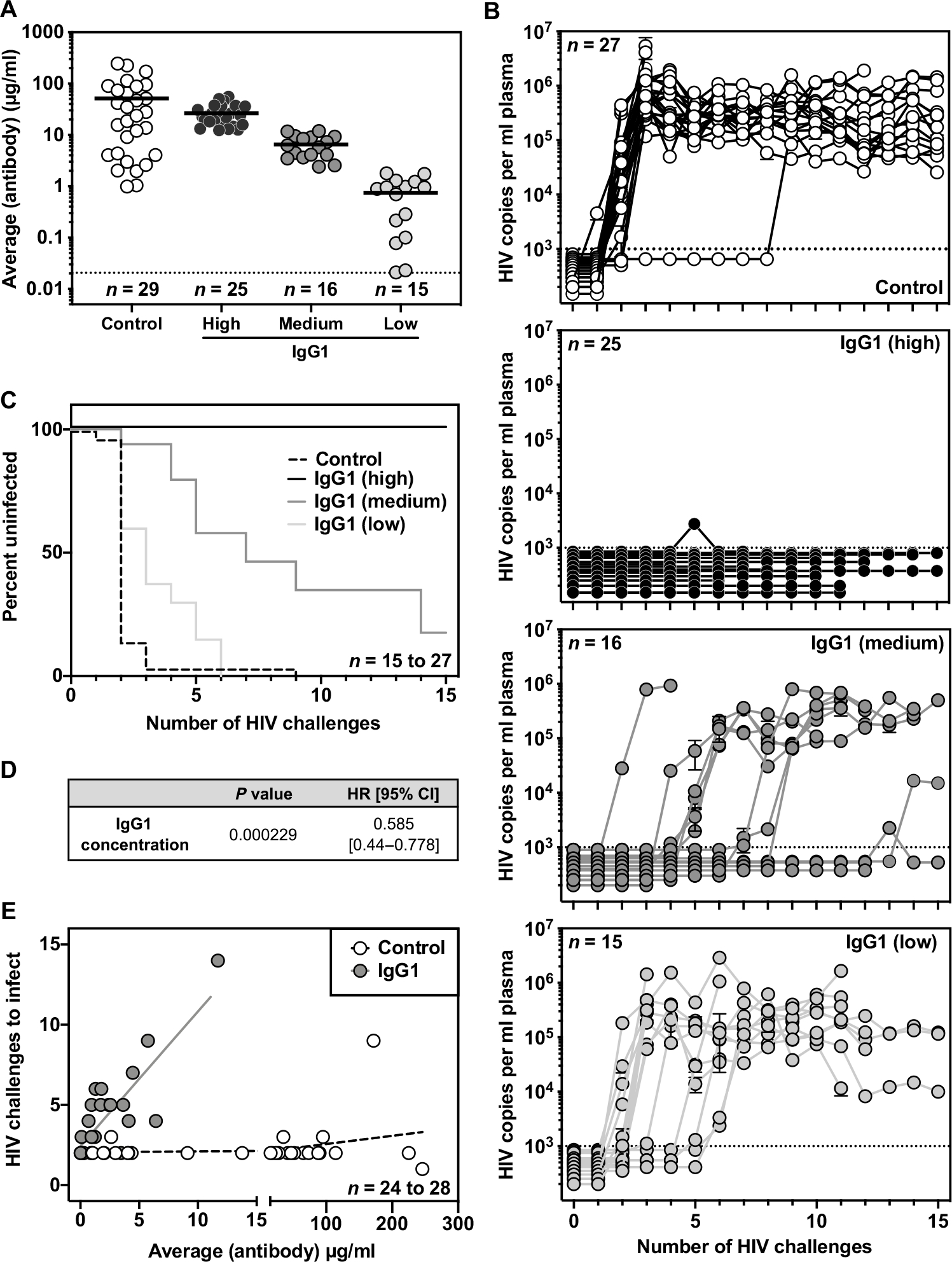
(A) Average plasma antibody concentrations achieved in BLT mice from the start of vaginal challenge through HIV acquisition or death are shown. Mice given AAV-VRC07-IgG1 were grouped on the basis of the average plasma VRC07 concentration achieved over the course of challenge. High, greater than 12 μg/ml; medium, 2 to 10 μg/ml; low, less than 2 μg/ml. Malaria-specific negative control IgG1 antibody concentrations were determined using a separate ELISA protocol. The graphs represent combined data from three separate BLT experiments. Horizontal bars indicate the mean. The horizontal dotted line indicates the limit of detection, 0.02 μg/ml, for the assay. (B) Viral load in the peripheral blood of BLT mice was measured by qPCR. The dotted line indicates the limit of detection (1000 copies/ml) for the assay. (C) Kaplan-Meier survival curves are shown for mice expressing a malaria-specific control IgG1 antibody or varying concentrations of VRC07 IgG1. (D) Cox regression analysis was used to evaluate the effect of VRC07 IgG1 concentration on the rate of HIV acquisition across multiple BLT experiments. (E) The relationship between the average circulating antibody concentration (micrograms per milliliter) and the number of HIV challenges required for HIV infection to occur over the course of challenge in BLT humanized mice was plotted. Lines represent the result of a linear regression for mice expressing VRC07 IgG1 (n = 24 mice) or a malaria-specific negative control IgG1 (n = 28 mice).
