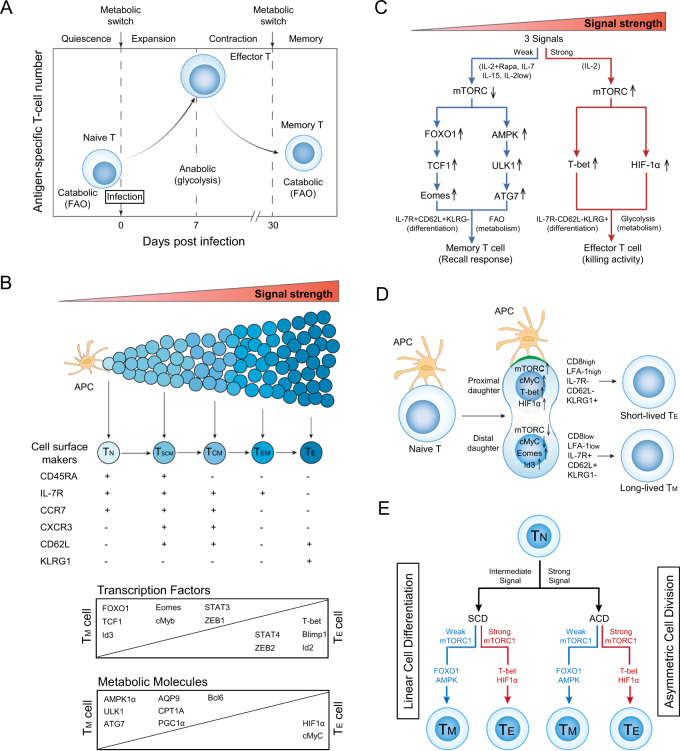
Fig. 1.
mTORC1 signaling with distinct strengths controls T-cell memory via the transcriptional FOXO1 and metabolic AMPKα1 pathways in both the linear cell differentiation and asymmetric cell division models. A Metabolic changes in CD8+ T cells at various stages of an acute infection. In response to a pathogenic stimulus, naïve CD8+ T cells enter a developmental program characterized initially by T-cell expansion and then by a subsequent contraction phase. During this latter phase, the majority of effector T (TE) cells undergo apoptosis, while the remaining minority of T cells differentiate into long-lived memory T (TM) cells. To meet the bioenergetic demand during the expansion phase, naïve T cells switch from mitochondrial respiration to glycolysis. During contraction to the memory phase, the metabolic program reverts to catabolic fatty acid oxidation as TE cells gradually transition into TM cells. B In the linear cell differentiation model, signals provided at distinct strengths by the antigen-presenting cell (APC) control naïve T-cell differentiation into short-lived TE and long-lived TM cells, including stem cell-like TM (TSCM), central TM (TCM), and effector TM (TEM) cells. The phenotypic attributes and expression levels of transcription factors and metabolic molecules controlling these cellular phenotypes are illustrated. C Schematic diagram of the distinct strengths of cytokine signaling (strong and weak) that control naïve T-cell differentiation into TE and TM cells. A strong cytokine (IL-2, regular dose 100 U/ml) signal stimulates strong mTORC1 (mTORC1Strong) signaling, leading to the formation of TE cells via transcriptional T-bet and metabolic HIF-1α pathways. In contrast, weak cytokine [IL-7, IL-15, low dose (10 U/ml) of IL-2 (IL-2Low) and IL-2 (regular dose) plus rapamycin (IL-2+Rapa)] signals stimulate weak mTORC1 (mTORC1Weak) signaling, leading to the formation of TM cells via transcriptional FOXO1-TCF1-Eomes and metabolic AMPK-ULK1-ATG7 pathways. D In the asymmetric cell division model, the proximal CD8HighLFA-1HighKLRG1+IL-7R-CD62L- daughter cell displays upregulation of mTORC1, cMyC, T-bet, and HIF-1α and adopts a CD8+ TE-cell fate during the first cell division. The distal CD8LowLFA-1LowKLRG1-IL-7R+CD62L+ daughter cell shows up- and downregulation of Id3/Eomes and mTORC1/cMyC, respectively, and is destined to become a TM cell. The green region at the contact interface between the APC and engaged T cell represents the immunological synapse. E Schematic diagram illustrating in both the asymmetric cell division (ACD) model with ACD and linear cell differentiation (LCD) model with symmetric cell division (SCD), how the mTORC1Weak signal promotes T-cell memory via transcriptional FOXO1 and metabolic AMPK pathways and the mTORC1Strong signal induces TE cell formation via transcriptional T-bet and metabolic HIF-1α pathways