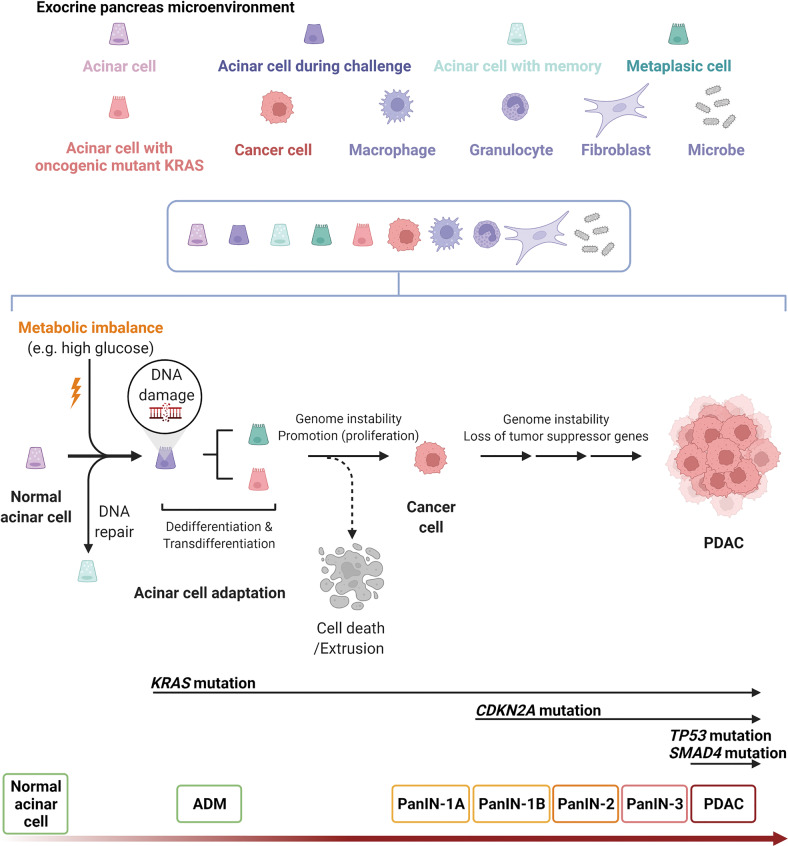
Fig. 4. Normal acinar cells undergo sustained transcriptional and epigenetic reprogramming after challenging metabolic imbalance (e.g., high glucose).
In response to DNA damage, cells may activate cell cycle checkpoints, process epigenetic and transcriptional programs or DNA repair (with epithelial memory), or undergo apoptosis when the damage is severe. Cells with adapted DNA mutation (e.g., oncogenic KRAS mutation) survived apoptosis and extrusion, with histologic and genetic progression, may transform into cancer cells and further grow as pancreatic ductal adenocarcinoma (PDAC) in adaptive to exocrine microenvironment-induced genome instability. Histologically, PDAC stems from microscopic abnormal acinar-to-ductal metaplasia (ADM) and pancreatic intraepithelial neoplasias (PanINs) of grades I–III to PDAC with increasing disorganization and nuclear abnormalities. High percentages of patients with PDAC carry somatic mutations, including KRAS proto-oncogene, GTPase (KRAS; ~95%) cyclin-dependent kinase inhibitor 2A (CDKN2A; ~90%), tumor protein 53 (TP53; ~70%), and SMAD family member 4 (SMAD4; ~55%). The pancreatic exocrine microenvironment is composed of exocrine cells at different stages, fibroblasts, immune cells, and microbes. The role of these microenvironmental components in adaptation, cancer initiation and progression remains to be elucidated. The graph was created with BioRender.com.