Table 7.
Nucleophilic cleavage of N-acyl ureas (activated N-methyldiaminobenzoyl linker)
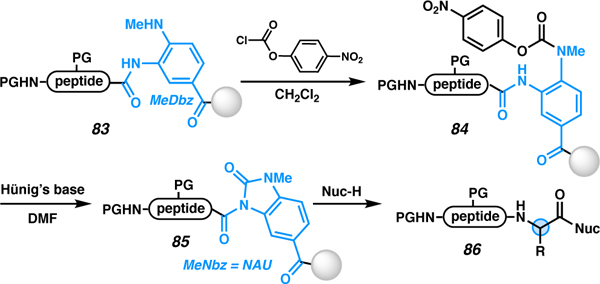
| ||||
|---|---|---|---|---|
|
| ||||
| entry | Peptidea | Nuc-H | % conversion (% isolated yield) | epimerization (% D-AA)b |
| 1 | AWG | NH3 | >99 (41) | n.a. |
| 2 | AWG |

|
>99 (30) | n.a |
| 3 | AWG | MeOH | >99 (41) | n.a |
| 4 | AWI | NH3 | >99 (68) | n.i. |
| 5 | AWA | BuNH2 | >99 | <1 |
| 6c | AWA | H2O | 70 | <1 |
| 7 | AWH(Trt) | NH3 | – | <1 |
| 8 | AWC(Trt) | NH3 | >99 | <1 |
| 9 | AWC(Trt) | BuNH2 | >99 | <1 |
| 10 | AWC(Trt) | PhCH2NH2 | >99 | <1 |
| 11d | AWC(Trt) | MeOH/Na2H PO4 | >99 | <1 |
| 12c | AWC(Trt) | H2O | 56 | <1 |
Trp (W) was Boc protected
n.d. = not determined, n.i. = not investigated
Hünig’s base was used to form the hydroxide species
an aqueous buffer of NaH2PO4/Na2HPO4 at pH 8 was used as co-solvent.
