Figure 3.
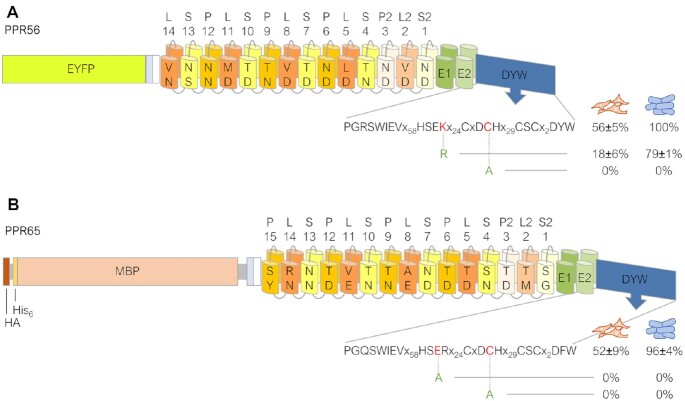
Mutations in the DYW domains of PPR56 and PPR65 result in strong reduction of editing. Constructs EYFP-PPR56 (A) and HA-His6-MBP-PPR65 (B) were selected for mutating key residues in their respective DYW domains. Exchanging the highly conserved cysteine in the DCH motif for alanine abolishes editing activity of both factors completely. Likewise, replacing the highly conserved glutamate in the HSER motif with alanine destroys editing activity of PPR65. The conservative exchange of lysine by arginine in the HSEK motif of PPR56 reduces editing from 56% to 18%. RNA editing efficiencies and standard deviations are shown for at least three replicates each (in case of absence of editing for at least two replicates). For a complete list of results from individual replicates see Supplementary Table S2. The effects of the corresponding mutations observed in the E. coli setup with recombinant His6-MBP tagged PPR proteins are indicated to the right (blue cell icons) of the human cell (orange cell icons) data.