Abstract
Purpose
Anosmia or hyposmia, with or without taste changes, are common symptoms that occur in SARS-CoV-2 infection and frequently persist as post-COVID-19 manifestations. This is the first trial to assess the potential value of using local ivermectin in the form of a mucoadhesive nanosuspension nasal spray to treat post-COVID-19 anosmia.
Methods
It is a controlled, randomized trial. Participants were recruited from South Valley University Hospitals in Qena, Upper Egypt, from the ENT and Chest Diseases Departments and outpatient clinics. Patients with persistent post COVID-19 anosmia were randomly divided into two groups, the first group “ivermectin group” included 49 patients treated by ivermectin nanosuspension mucoadhesive nasal spray (two puffs per day). The second group included 47 patients “placebo group” who received saline nasal spray. Follow- up of anosmia [using Visual analogue scale (VAS)] in all patients for three months or appearance of any drug related side effects was done.
Results
The mean duration of pre-treatment post COVID-19 anosmia was 19.5± 5.8 days in the ivermectin group and 19.1± 5.9 days in the placebo group,p˃0.05. Regarding the median duration of anosmia recovery, the ivermectin group recovered from post COVID-19 anosmia in 13 days compared to 50 days in the placebo group, p˂ 0.001. Following the first week of ivermectin nanosuspension mucoadhesive nasal spray therapy, the ivermectin group had a significantly higher percentage of anosmia recovery (59.2%) than the placebo group (27.7%), p˂ 0.01, with no significant differences in recovery rates between the two groups at 1, 2, and 3 months of follow up, p˃0.05.
Conclusion
In the small number of patients treated, local Ivermectin exhibited no side effects. In persistent post-COVID-19 anosmia, it could be used for one week at the most as the treatment was extended to one, two and three months, with no difference in recovery compared to the placebo treatment.
Trial Registration No
Keywords: post-COVID-19 anosmia, ivermectin, nanosuspension mucoadhesive nasal spray
Introduction
The pandemic of coronavirus disease 2019 (COVID-19) started in December 2019 in China. It is a viral disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections.1,2 Patients with COVID-19 could be presented with fever, fatigue, headache, cough and dyspnea.3 In severe cases, patients suffer from pneumonia, acute respiratory failure, respiratory distress syndrome, and acute heart problems.4
Anosmia or hyposmia, with or without problems in taste, is a common symptom that occurs in SARS-CoV-2 infection.5,6 The frequency of olfactory dysfunction in COVID-19 ranges from 68 to 85%, and it may persist for weeks post COVID-19.5 Carfi et al, reported that 87.4% of patients who recovered from COVID-19 had at least one persistent symptom with loss of smell among them.7 However, the underlying mechanism by which anosmia develops itself has not yet been identified.8 There are several theories that explain how anosmia occurs in COVID-19 patients. It is claimed that coronaviruses invade the olfactory pathway through the first order neuron of the central nervous system which presents in the olfactory mucosa. This olfactory mucosa is located in the roof of the nasal cavity and is formed of a specialized neuro-epithelium.9 The virus reaches the second order neuron by crossing the cribriform plate, and then reaches the olfactory bulb.10 One potential mechanism for the anosmia brought on by COVID-19 is olfactory epithelium (OE) inflammation. The virus’s attachment to these cells results in cytokine production, which encourages OE inflammation. In one study, IL-6 levels and olfactory abnormalities in COVID-19 patients were compared. Significant relationships between the lowered levels of IL-6 and the length of time needed to recover from anosmia brought on by COVID-19 have been reported.11 Ivermectin interferes with STAT-3 function and reduces IL-6 production via inhibiting Akt/mTOR signalling and promoting PAK1’s ubiquitin-mediated degradation.12 The reduction of cytokine production by lipopolysaccharide-exposed macrophages, blocking of NF-kappa B activation and the stress-activated MAP kinases JNK and p38, and inhibition of toll-like receptor 4 (TLR4) signalling were proposed as the mechanism for ivermectin’s anti-inflammatory activity.13–15 Anosmia is a distressing symptom that may disturb quality of life; numerous trials were conducted to treat it. According to Abdelmaksoud et al, zinc therapy may significantly shorten the time it takes for COVID-19 patients to restore their sense of smell.16
Ivermectin was previously used in treatment of Onchocerca volvulus and lymphatic filariasis.17 It is a well-known antiviral drug effective against many viruses as confirmed by many in vitro studies.18,19 Ivermectin has been reported to inhibit the nuclear import of viral proteins; it has also been proved to limit the infection by RNA viruses such as dengue, West Nile and influenza viruses. SARS-Cov-2 viral RNA replication is reduced about 5000- fold at 48 hours by ivermectin in an in vitro study.20,21
Nanosuspensions are sub-micron colloidal dispersions of pure drug dispersed in an aqueous medium in which the diameter of the suspended particles ranges from 10 to 1000 nm).22 These particles can be stabilized by adding a minimal amount of polymers, surfactants, or a combination of the two. Nanosuspensions have high drug loading capacity, lower toxicity, and high chemical stability.23 For intranasal drug administration, the drug must remain in the nasal cavity for a prolonged period of time in order to be absorbed.22 Therefore, mucoadhesive formulations must be added to these nanoparticles to maintain their localization inside the nasal cavity for longer duration.23 Aref et al used an ivermectin mucoadhesive nanosuspension nasal spray to treat early COVID-19 patients, and they found that this innovative delivery approach is efficient and safe for treating mild COVID-19 patients and shortens anosmia duration.24 In addition to mucoadhesive material, the goal of this study was to assess the potential role of Ivermectin when administered locally as a nanosuspension nasal spray in the treatment of individuals with post-COVID-19 anosmia.
Patients and Methods
Study Design and Participants
The randomized controlled trial was conducted on patients presented with post COVID-19 anosmia recruited from the ENT and chest diseases’ Departments and out-patients from clinics of hospitals of South Valley University, Qena, Upper Egypt, during the period from March 1st, 2021 to April 30th, 2021. The Declaration of Helsinki was followed in conducting the study. The South Valley University Faculty of Medicine’s Ethics Committee gave their approval to this study. Code: (SVU 2021/1/121). Informed written consent for inclusion in this study was obtained from each participant in the study. If the participants were under 18 years of age, their informed written consent was obtained from their parents or legal guardians.
Inclusion Criteria
Patients with COVID-19 anosmia and persists after viral clearance (confirmed by negative two successive SARS-CoV-2 RNA-PCR nasopharyngeal swabs, 48 hours apart), and approved to be included in the study.
Exclusion Criteria
Participants were excluded if they had previously been diagnosed with nasal polyps, undergone anterior or sinonasal surgery, or had a neurological condition prior to COVID-19 infection. Individuals who were pregnant or lactating were not included. Additionally, people were excluded if they were concurrently taking other treatments for olfactory dysfunction, or had comorbid illnesses like asthma, or had an adverse reaction to ivermectin, or had any other conditions that would make it inappropriate for them to take ivermectin.
The diagnosis of anosmia was made by the physicians.16 COVID-19 anosmia patients were randomly divided into two groups; the first group consisted of 49 patients with post COVID-19 anosmia, treated by local Ivermectin in the form of nanosuspension mucoadhesive nasal spray (two puffs per day). The second group included 47 patients with post COVID-19 anosmia who received isotonic saline (NaCl 0.9%) nasal spray.
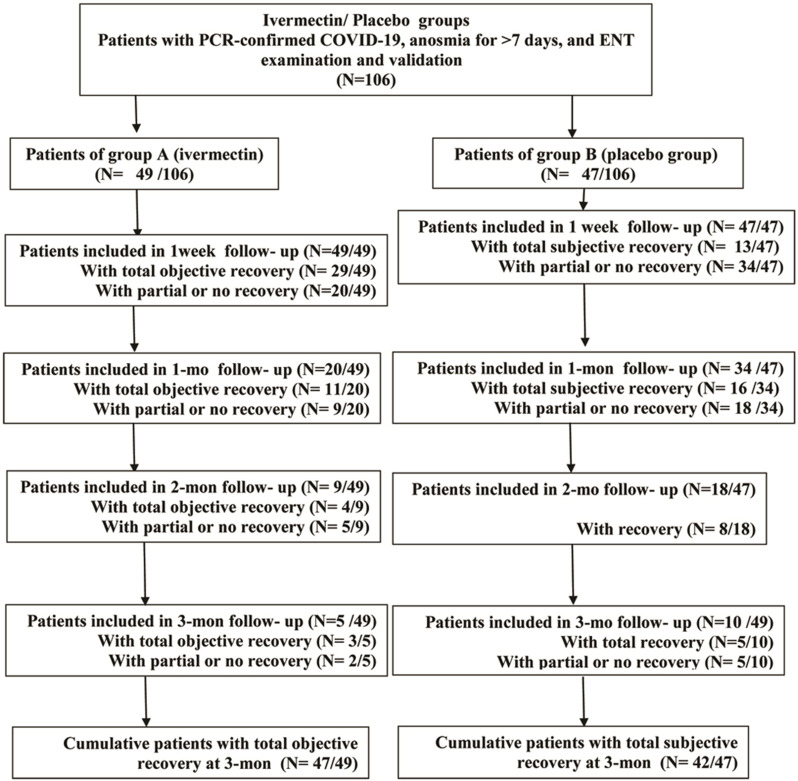
Although there have been numerous studies on the olfactory dysfunction that COVID-19 patients experience, post-COVID-19 anosmia has not yet been successfully treated.25 Isotonic NaCl irrigation has no hazard or therapeutic effect on anosmia.26 Objective and subjective assessments of anosmia recovery were performed for three months follow- up of all included participants at four different times (one week, one month, two months, three months intervals), Figure 1. The results were recorded in days. Patients were randomly assigned (1:1) either to; Ivermectin nanosuspension mucoadhesive nasal spray or saline nasal spray. In an electronic case report form system, randomization was carried out in blocks of varying sizes (4, 6, and 8), stratified by age (≥25 years vs <25 years). Allocation was done by a web-based, automated randomization system but the study was open label without masking. Each participant was given written, verbal, and visual instructions on how to carry out the nasal irrigation to ensure patients’ adherence to use the prescribed medication.
Figure 1.
Flow chart showing difference between both group in different follow up durations.
Sample size was calculated using the Steven K. Thompson equation. We adjusted the sample size to attain 80% power and a 5% confidence level of significance (type I error).
Olfactory Assessments and Data Collection
To grade anosmia, a visual analogue scale (VAS) was utilized (from 0 to 10) with 0: no sense of smell, 10: normal sense of smell), and scores from 1 to 9 indicating progressively recovery of smell.27 Demographic data for all patients were recorded including sex, age and co morbidities as (DM and HTN). For each patient included, a comprehensive history and clinical examination were performed. In addition, local nasal examination of the nose and paranasal sinuses was performed to exclude any causes of nasal obstruction.
Materials Used for Ivermectin Preparation
Sodium alginate was purchased from General Chemical and Pharmaceuticals Co. Ltd, Sudbury, Middleesex, England. Hydroxy propyl methyl cellulose (HPMC) 15,000 was purchased from El-Gomhouria Co., Egypt. Pluronic-F127 and Pluronic-F68 were purchased from Sigma Chemicals Co. (St. Louis, MO).
Preparation of Ivermectin Nanosuspension
Ivermectin nanosuspension was produced utilizing antisolvent precipitation followed by ultrasonication methods.19,23 Ivermectin was dissolved in acetone in a specific concentration (120 mg/L). Pluronic-F68 and Pluronic-F127 at concentrations of 1 and 2% w/v, respectively, were dissolved in distilled water to form a stabilizer aqueous phase. The prepared ivermectin solution was then added slowly (dropwise) using a syringe to the stabilizer aqueous solution under continuous stirring on a magnetic stirrer (3000 rpm for 30 minutes) at room temperature. Immediately after mixing, precipitation occurred, forming a homogeneous suspension, and then it was subjected to ultrasonication using a probe-type sonicator (Cole-Parmer, Vernon Hills, IL) for 10 minutes at 5 second pulses and 50% amplitude pressure. The formed nanosuspension was placed on a magnetic stirrer to ensure evaporation of organic solvent. The entrapment efficiency (EE %) of the prepared formulation was evaluated indirectly by estimating the unentrapped ivermectin spectrophotometrically at 245 nm using this equation:28
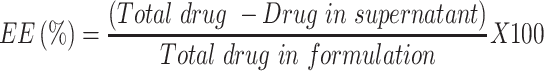 |
Particle size (nm) and polydispersity index (PDI) were determined by dynamic light scattering using a Zetasizer Nano ZS® instrument (Malvern Instruments, Malvern, UK). The developed ivermectin nanosuspension was imaged using transmission electron microscopy (JEOL TEM, Model 100 CX II; Tokyo, Japan).
Ivermectin Nanosuspension Mucoadhesive Nasal Spray Preparation
Ivermectin nanosuspension was obtained by adding sodium alginate (0.2% w/v), carbopol 974P (0.1% w/v), and HPMC K 15M (0.3% w/v) and stirring until a homogeneous dispersion was obtained. Preservatives were then added to the ivermectin nanosuspension in the form of glycerol (1% w/v) and sodium benzoate (0.01% w/v). Finally, mucoadhesive nanosuspension was used to fill nasal spray bottles with a final concentration of ivermectin of 70 μg/mL.24,29 Drug content and the pH of the developed mucoadhesive nanosuspension were also, determined. The in-vitro release of ivermectin in simulated nasal fluid (SNF) from the prepared formulation was performed using dialysis method through cellophane membrane as mentioned previously.30 Release data were analyzed using different kinetic models (Zero order, First order, Higuchi diffusion model and Korsmeyer-Peppas).22 Finally, the storage stability of ivermectin nanosuspension at two different storage conditions, room temperature and refrigerated conditions (4°C) for one month.
Statistical Analysis
Data entry and data analysis were done using SPSS version 26 (Statistical Package for Social Science). Kolmogorov–Smirnov test was used to check for data normality. Data were presented as a number, percentage, the mean and standard deviation for parametric data, the median and inter-quartile range for non-parametric data. Chi-square and Fisher’s exact tests were used to compare qualitative variables. Mann–Whitney’s test was used to compare between two quantitative variables and Kruskal–Wallist’s test and Dunn’s post hoc test were used to compare between more than two quantitative variables for non-parametric data. Independent t test was used to compare between two quantitative variables for parametric data. Level of significance was considered when <0.05.
Results
Characteristics of the Prepared Ivermectin Mucoadhesive Nanosuspension Nasal Spray
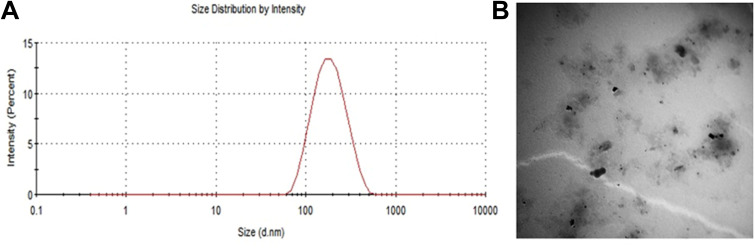
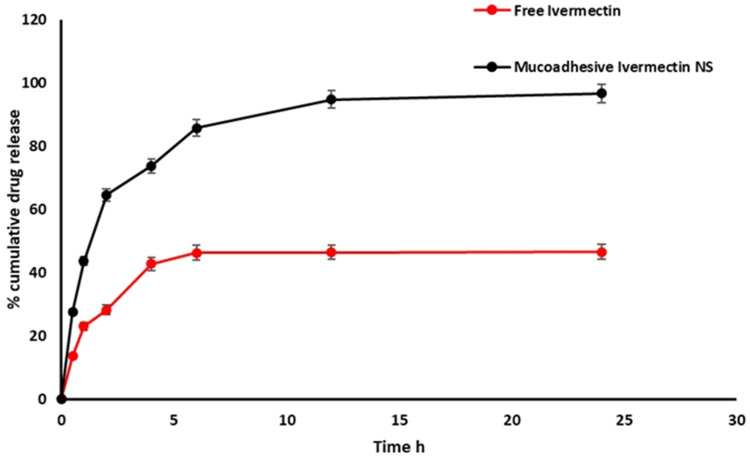
Ivermectin nanosuspension was developed successfully using anti-solvent evaporation ultrasonication method. The prepared formulation showed small particle size of 373.0 ± 16.38 nm (Figure 2A), acceptable PDI (polydispersity index) of 0.496 ± 0.086 (narrow size distribution),drug encapsulation of 96.9±2.3% with high zetapotential −31.0 ± 2.15mV. Transmission electron microscope (TEM) analysis revealed that the prepared ivermectin nanosuspension formulation has nonaggregate small sized with slightly spherical shape particles (Figure 2B). The cumulative release of ivermectin after 6 h from mucoadhesive nanosuspension was higher than (85.7 ± 0.82%) that from free ivermectin solution (46.23 ± 1.8%.) (Figure 3). This result might be attributed to the reduced particle size (373.0 ± 16.38 nm), improved aqueous solubility and high surface area of drug nanosuspension. Further, prolonged drug release was exhibited from mucoadhesive nanosuspension. Kinetic analysis of release data revealed that the release of ivermectin follows First-order kinetics (R2=0.928) and Korsmeyer-Peppas diffusional exponent n (0.663) corresponding to anomalous or non-Fickian diffusion.
Figure 2.
Characteristics of the used ivermectin nanosuspension. (A) Particle size distribution. (B) Transmission electron micrograph of the prepared ivermectin nanosuspension.
Figure 3.
In-vitro release profiles of ivermectin from mucoadhesive nanosuspension in SNF (simulated nasal fluid) pH 5.5 at 37 °C. Data expressed at mean ± SD (n=3).
Abbreviation: NS, Nanosuspension.
Demographic and Clinical Data of the Study Groups
This study included 96 patients with post COVID-19 Anosmia. None of them were chronically use alcohol or opiates. Patients were divided randomly into two groups; group A consisted of 49 patients with a mean age of 30.9 ± 14 years (age range 16–44 years) versus 47 patients of group B with a mean age of 29.1± 9.6 years (age range 19–40) with no significant difference. Both groups were matched according to gender, p ˃0.05 (Table 1). In the context of co-morbidities, four patients were diabetic in group A and two patients were hypertensive, and two patients were diabetics and hypertensive. In group B also two patients were hypertensive, and two patients were diabetics and hypertensive with no significant differences between both groups regarding to the frequency percentages of the co-morbidities, p˃0.05 for all (Table 1). According to the severity of COVID-19 in group A; mild cases were 29 patients (59.2%), moderate cases were 11 patients (22.4%) and severe cases were 9 patients (18.4%). In group B mild cases were 30 patients (63.8%), moderate cases were 9 patients (19.1%),and severe patients were 8 (17%) with no significant difference between both groups, p˃0.05 for all (Table 1). Regarding to the anosmia duration before starting treatment, it was 19.5± 5.8 days in group A and 19.1± 5.9 in group B with p˃0.05 for all (Table 1).
Table 1.
Demographic and Clinical Data of the Study Groups
| Variables | Ivermectin Group (n= 49) | Placebo Group (n=47) | P value | |
|---|---|---|---|---|
| Age (years, mean±SD) | 30.9 ± 14 | 29.1± 9.6 | 0.5 | |
| Sex (No.,%) | Males | 33(67.3%) | 39(83%) | 0.07 |
| Females | 16(32.7%) | 8(17%) | ||
| Co-morbidities (No.,%) | No | 41(83.7%) | 39(83%) | 0.8 |
| Diabetes mellitus | 4(8.2%) | 4(8.6%) | ||
| Hypertension | 2(4.1%) | 2(4.3%) | ||
| Diabetes mellitus and hypertension | 2(4.1%) | 2(4.3%) | ||
| COVID-19 severity (No.,%) | Mild | 29(59.2%) | 30(63.8%) | 0.9 |
| Moderate | 11(22.4%( | 9(19.1%) | ||
| Severe | 9(18.4%) | 8(17%) | ||
| Anosmia duration before starting therapy(days, mean±SD) | 19.5±5.8 | 19.1±5.9 | 0.7 | |
Abbreviations: SD, standard deviation; COVID-19, Coronavirus disease 2019.
Post-COVID-19 Anosmia Recovery Percentages at Different Points of Follow Up Duration Among the Study Groups
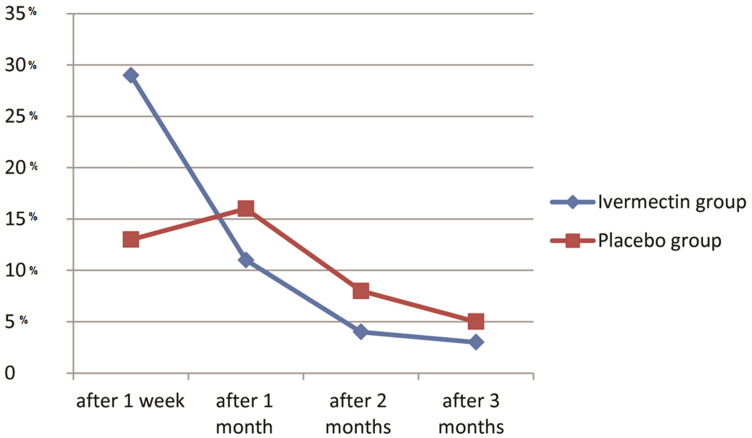
After one week of treatment the ivermectin group (A) showed recovery in 29 patients (59.2%). The placebo group (B) showed recovery in 13 patients (27.7%) with highly statistically significant difference (p ˂ 0.01). After one month, duration eleven patients (55%) in group A recovered, and 16 patients recovered in group B (47.1%) with no statistically significant difference (p˃0.05). After two months follow-up, 4 patients in group A recovered (44.4%), and 8 patients (44.4%) recovered in group B with p˃0.05 which was not statistically significant. After 3 months follow-up 3 patients (60%) recovered in group A and 5 patients (50%) recovered in group B, with no statistically significant difference. The cumulative recovered patients in the ivermectin group were 47 patients (95.9%) and 42 patients (89.4%) in the placebo group with no statistically significant difference (Table 2, Figures 1 and 4).
Table 2.
Comparison Between the Study Groups Regarding to Anosmia Recovery at Different Follow Up Durations
| Follow Up (No.,%) | Ivermectin Group (n= 49) | Placebo Group (n=47) | P value | |
|---|---|---|---|---|
| 1 week | Recovered | 29(59.2%) | 13(27.7%) | 0.001* |
| Partially or not recovered | 20(40.8%) | 34(72.3%) | ||
| 1 month | Recovered | 11(55%) | 16(47.1%) | 0.6 |
| Not recovered | 9(45%) | 18(52.9%) | ||
| 2 months | Recovered | 4(44.4%) | 8(44.4%) | 0.99 |
| Not recovered | 5(55.6%) | 10(55.6%) | ||
| 3 months | Recovered | 3(60%) | 5(50%) | 0.7 |
| Not recovered | 2(40%) | 5(50%) | ||
| Cumulative recovered patients | 47(95.9%) | 42(89.4%) | 0.2 | |
Note: *Significant p value (p˂0.05).
Figure 4.
Comparision between the study groups according to the percentage (%) of recovery of anosmia in different follow up durations (after one week, one month, two months and three months).
Comparison of Total Duration of Post-COVID-19 Anosmia Till Recovery in the Study Groups
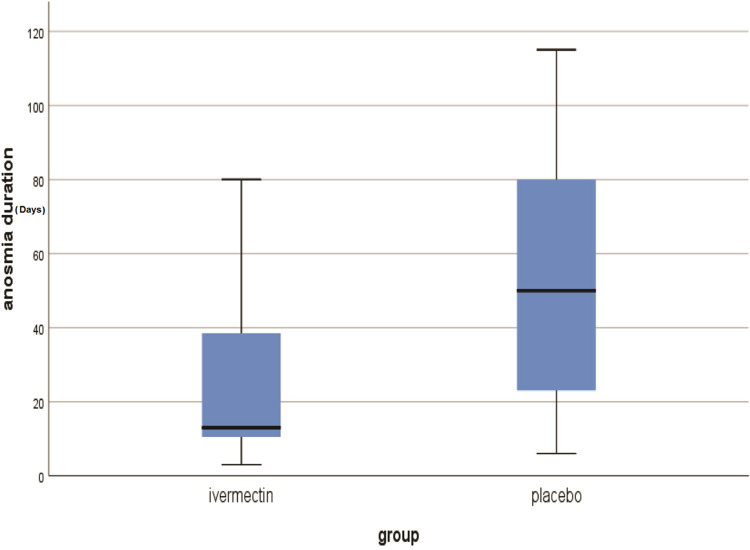
The median and range of duration of anosmia till recovery in group A was 13±(3–105) days versus 50±(6–115) in group B with a highly statistically significant difference (Table 3, Figure 5).
Table 3.
Comparison Between the Total Duration of Anosmia Till Recovery in the Study Groups
| Variable | Ivermectin Group (n= 49) | Placebo Group (n=47) | P value |
|---|---|---|---|
| Duration of anosmia till recovery [days, Median (Interquartile range)] | 13 (3–105) | 50 (6–115) | 0.000* |
Notes: Mann Whitney test was used. *Significant p value (p˂0.05).
Figure 5.
The median duration of post-COVID-19 persistent anosmia recovery (days) in the study groups.
This current study reported no side effects in any patient treated with Ivermectin mucoadhesive nanosuspension nasal spray (headache, dizziness, muscle pain, nausea or diarrhea).
Discussion
Ivermectin nanosuspension nasal delivery spray ensures homogeneous medication localization via the nasal mucosa. Hydroxypropyl methylcellulose (HPMC K 15M), Carbopol 974P and Sodium Alginate are mucoadhesive polymers and they are used in a blend mixture to prolong the presence of the drug at the site of administration. Consequently, the dose frequency may be decreased. Based on the previously mentioned points, the aim of this study was to formulate ivermectin nanosuspension into a mucoadhesive nasal spray in order to evaluate the possible ivermectin therapeutic activity in treatment of post COVID-19 persistent anosmia. The effectiveness of ivermectin in COVID-19 patients currently lacks a comprehensive and current evidence synthesis. To evaluate clinical outcomes, numerous clinical trials were conducted, however the results were inconsistent. Several clinical trials have demonstrated no benefit of ivermectin compared to placebo for treatment of COVID-19.31,32 While other trials support its beneficial role.33,34
Patients with COVID-19 complain of sudden onset of anosmia without rhinitis as a new symptom and may be due to intranasal inoculation of SARS-CoV-2 into the olfactory neural circuitry.35 Gane et al revealed that sudden anosmia might be the only symptom present in COVID-19 patients.6 Examination of autopsy specimens from COVID-19 patients revealed a neuro-invasion of coronavirsues. Coronavirus strains HCoV-OC43 and −229E were detected in CNS postmortem specimens.36,37 Cerebrospinal fluid has revealed SARS-CoV-2 was detected in the cerebrospinal fluid and also in CNS postmortem specimens.38,39 Another possible way of viral entry into the CNS is by direct brain inoculation from epithelial disruption at the blood-brain barrier after hematologic seeding of SARS-CoV-2 from other organs. The lung and intestinal epithelia showed high prevalence of ACE2, which supports the hematologic routes for viral entry.40
No certain drug therapy for post COVID-19 persistent anosmia has been documented until now. Ivermectin is a drug that has received FDA approval.20 It has been evaluated for the treatment of COVID-19 in several clinical studies worldwide.41 Ivermectin can inhibits replication of a number of viruses in vitro.18,19 In an in vitro study, Ivermectin was found to be an inhibitor of the SARS-CoV-2, two hours post infection when added to Vero/ hSLAM cells and able to reduce ~5000-fold viral RNA levels at 48 hours.20,21 In a recently published study, we reported that an Ivermectin mucoadhesive nanosuspension nasal spray showed significant rapid recovery of mild COVID-19 patients with shorter duration of upper respiratory symptoms mainly anosmia. Also, the treatment was reported that it was safe with no side effects.24 However, this study was performed on patients with active COVID-19 and this led us to use this preparation in the current study for its possible role in recovery of anosmia that persists after viral clearance, even though further studies on the potential mechanism are required. Nanoparticles comprise a carrier-free colloidal drug delivery system with a mean particle size in the nanometer range, containing only pure drug and minimum surfactant and/or polymer for stabilization. Nanosuspensions are able to improve solubility and bioavailability of poorly soluble drugs.22
In this study we used polymers with strong mucoadhesive capacity, which can significantly limit the total clearance of the formulation from the nasal cavity; thus, it can provide prolonged retention and increased bioavailability of the drug. Bioadhesive polymers present in the mucoadhesive drug delivery systems, becomes adhesive in nature upon hydration and therefore find perspectives for targeting the drug(s) to specific body locations to extend the therapeutic effect.42 Nasal delivery of nanosuspensions has been investigated in different studies. A nasal spray formulation based on in situ gel containing carvedilol nanosuspension increased the absolute bioavailability in comparison with orally administered carvedilol, which prolonged residence time.43 The nasal nanosuspension loratadine showed a significantly improved bioavailability.22 Therefore, in-vitro release of ivermectin was performed in simulated nasal fluid (SNF) to investigate the effect of mucoadhesive nanosuspension on release pattern of ivermectin. The presence of mucoadhesive polymers form a thick gel layer that allow the drug to be released in sustained release manner which is controlled by polymer erosion and diffusion mechanisms.43 Prepared nanosuspension in the current study showed physicochemical properties indicates the homogeneity, high drug yield, improved ivermectin aqueous solubility34 and good stability.43 The pH of preparation was found to be 5.8 which is within the acceptable range for nasal administration (pH of the nasal mucosa is 4.5–6.5).34 The results of storage stability studies revealed that the prepared nanosuspension showed higher stability at temperature of 4 ±2°C, the change in particle size, PDI and EE% (380.54 ± 21.20 nm, 0.500±0.036 and 95.84±1.2%) was negligible. These values were in acceptable ranges which prove the physical stability of examined formulation at 4 ±2°C for one month. However, it was found that the examined parameters are changed slightly at 25±2°C. This finding suggests that it is preferable to store the prepared formulation in refrigerator.
The results of this study showed a significant reduction in the duration of recovery of patients with persistent post-COVID-19 anosmia. Also, no side effects were reported. In this study patients with persistent post-COVID-19 anosmia treated by local ivermectin in the form of nanosuspension mucoadhesive nasal spray showed statistically significant improvement in the first week of treatment in comparison to the placebo group of patients. However, this statistically significant improvement with ivermectin mucoadhesive nanosuspension nasal spray was not reported in patients at one, two and three months of treatment. The effect of ivermectin nanosuspension mucoadhesive nasal spray in the treatment of post COVID-19 persistent anosmia may be attributed to the direct virucidal effect of ivermectin on persistent viral particles or virions on the nasal mucosa and olfactory bulb, although further molecular research to highlight the mechanism of local ivermectin in recovery of post-COVID-19 anosmia is required.
Study’s Limitations
Relatively small sample size, use of subjective assessment of anosmia and lack of comparison with a third therapeutic regimen such as nasal corticosteroids were the main limitations of the current study. Additionally the use of the visual analogue scale (VAS) for grading anosmia has its limitations and some patients may not answer correctly was also another study limitation.
Conclusion
The result of this study showed that the overall recovery rate of patients from post-CVID-19 anosmia was not significant in the ivermectin group; however, in the intervention group patients recovered faster than the control group after one week of therapy. However, the treatment was extended to one, two and three months, which showed no difference to placebo treatment. Further multicenter studies need to evaluate the clinical effects of ivermectin mucoadhesive nanosuspension nasal spray in treating post-covid-19 persistent anosmia.
Funding Statement
This research was partially funded by South Valley University, Faculty of Medicine, Qena, 83523, Egypt.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for Publication
Not applicable because the manuscript lacked the names, identifiers, or images of the patients.
Consent for Participation
After thorough explanation of the study’s objectives and procedures, informed written consent was obtained from each participant in the trial, or from relatives if the patients were less than 18 years old and those who supplied consent on their behalf were their parents or legal guardians.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in relation to this work.
References
- 1.World Health Organization. Coronavirus disease (COVID-2019) situation reports; 2020. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/. Accessed April 9, 2020.
- 2.Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19): cases in U.S; 2020. Available from: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed April 9, 2020.
- 3.Whittaker A, Anson M, Harky A. Neurological Manifestations of COVID-19: a systematic review and current update. Acta Neurol Scand. 2020;142(1):14–22. doi: 10.1111/ane.13266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss P, Murdoch DR. Clinical course and mortality risk of severe COVID-19. Lancet. 2020;395(10229):1014–1015. doi: 10.1016/S0140-6736(20)30633-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lozada-Nur F, Chainani-Wu N, Fortuna G, Sroussi H. Dysgeusia in COVID-19: possible Mechanisms and Implications. Oral Surg Oral Med Oral Pathol Oral Radiol. 2020;130(3):344–346. doi: 10.1016/j.oooo.2020.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gane SB, Kelly C, Hopkins C. Isolated sudden onset anosmia in COVID-19 infection. A novel syndrome? Rhinology. 2020;58(3):299–301. doi: 10.4193/Rhin20.114 [DOI] [PubMed] [Google Scholar]
- 7.Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients After Acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaira LA, Salzano G, Fois AG, Piombino P, De Riu G. Potential pathogenesis of ageusia and anosmia in COVID-19 patients. Int Forum Allergy Rhinol. 2020;10(9):1103–1104. doi: 10.1002/alr.22593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fodoulian L, Tuberosa J, Rossier D, et al. SARS-CoV-2 Receptors and Entry Genes Are Expressed in the Human Olfactory Neuroepithelium and Brain. iScience. 2020;23(12):101839. doi: 10.1016/j.isci.2020.101839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Riel D, Verdijk R, Kuiken T. The olfactory nerve: a shortcut for influenza and other viral diseases into the central nervous system. J Pathol. 2015;235(2):277–287. doi: 10.1002/path.4461 [DOI] [PubMed] [Google Scholar]
- 11.Cazzolla AP, Lovero R, Lo Muzio L, et al. Taste and Smell Disorders in COVID-19 Patients: role of Interleukin-6. ACS Chem. Neurosci. 2020;11:2774–2781. [DOI] [PubMed] [Google Scholar]
- 12.Dou Q, Chen H-N, Wang K, et al. Iver-mectin induces cytostatic autophagy by blocking the PAK1/Aktaxis in breast cancer. Cancer Res. 2016;76(15):4457–4469. doi: 10.1158/0008-5472.CAN-15-2887 [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Song Y, Xiong H. Inhibitory effects of ivermectin on nitric oxide and prostaglandin E2 production in LPS-stimulated RAW 264.7 macrophages. Int Immunopharmacol. 2009;9(3):354–359. doi: 10.1016/j.intimp.2008.12.016 [DOI] [PubMed] [Google Scholar]
- 14.Ci X, Li H, Yu Q. Avermectin exerts anti-inflammatory effect by downregulating the nuclear transcription factor kappa-B and mitogen-activated protein kinase activation pathway. Fundam Clin Pharmacol. 2009;23(4):449–455. doi: 10.1111/j.1472-8206.2009.00684.x [DOI] [PubMed] [Google Scholar]
- 15.Ventre E, Rozières A, Lenief V. Topical ivermectin improves allergic skin inflammation. Allergy. 2017;72(8):1212–1221. doi: 10.1111/all.13118 [DOI] [PubMed] [Google Scholar]
- 16.Abdelmaksoud AA, Ghweil AA, Hassan MH, et al. Olfactory Disturbances as Presenting Manifestation Among Egyptian Patients with COVID-19: possible Role of Zinc. Biol Trace Elem Res. 2021;3:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.González Canga A, Sahagún Prieto AM, Diez Liébana MJ, Fernández Martínez N, Sierra Vega M, García Vieitez JJ. The pharmacokinetics and interactions of ivermectin in humans--a mini-review. AAPS J. 2008;10(1):42–46. doi: 10.1208/s12248-007-9000-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mastrangelo E, Pezzullo M, De Burghgraeve T, et al. Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug. J Antimicrob Chemother. 2012;67(8):1884–1894. doi: 10.1093/jac/dks147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Götz V, Magar L, Dornfeld D, et al. Influenza A viruses escape from MxA restriction at the expense of efficient nuclear vRNP import. Sci Rep. 2016;6(1):23138. doi: 10.1038/srep23138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 invitro. Antiviral Res. 2020;178:104787. doi: 10.1016/j.antiviral.2020.104787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ketkar H, Yang L, Wormser GP, Wang P. Lack of efficacy of ivermectin for prevention of a lethal Zika virus infection in a murine system. Diagn Microbiol Infect Dis. 2019;95(1):38–40. doi: 10.1016/j.diagmicrobio.2019.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alshweiat A, Csóka I, Tömösi F, et al. Nasal delivery of nanosuspension-based mucoadhesive formulation with improved bioavailability of loratadine: preparation, characterization, and in vivo evaluation. Int J Pharm. 2020;579:119166. doi: 10.1016/j.ijpharm.2020.119166 [DOI] [PubMed] [Google Scholar]
- 23.Alshweiat A, Ambrus R, Csoka I. Intranasal Nanoparticulate Systems as Alternative Route of Drug Delivery. Curr Med Chem. 2019;26(35):6459–6492. doi: 10.2174/0929867326666190827151741 [DOI] [PubMed] [Google Scholar]
- 24.Aref ZF, Bazeed SEES, Hassan MH, et al. Clinical, Biochemical and Molecular Evaluations of Ivermectin Mucoadhesive Nanosuspension Nasal Spray in Reducing Upper Respiratory Symptoms of Mild COVID-19. Int J Nanomedicine. 2021;16:4063–4072. doi: 10.2147/IJN.S313093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chong LY, Head K, Hopkins C, et al. Saline irrigation for chronic rhinosinusitis. Cochrane Database Syst Rev. 2016;4(4):CD011995. doi: 10.1002/14651858.CD011995.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yildiz E, Koca Yildiz S, Kuzu S, Günebakan Ç, Bucak A, Kahveci OK. Comparison of the Healing Effect of Nasal Saline Irrigation with Triamcinolone Acetonide Versus Nasal Saline Irrigation alone in COVID-19 Related Olfactory Dysfunction: a Randomized Controlled Study. Indian J Otolaryngol Head Neck Surg. 2021;1–6. doi: 10.1007/s12070-021-02749-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prajapati DP, Shahrvini B, MacDonald BV, et al. Association of subjective olfactory dysfunction and 12-item odor identification testing in ambulatory COVID-19 patients. Int Forum Allergy Rhinol. 2020;10(11):1209–1217. doi: 10.1002/alr.22688 [DOI] [PubMed] [Google Scholar]
- 28.Junior OV, Cardoso FAR, Giufrida WM, de Souza MF, Cardozo-Filho L. Production and computational fluid dynamics-based modeling of PMMA nanoparticles impregnated with ivermectin by a supercritical antisolvent process. J CO2 Util. 2020;35:47–58. doi: 10.1016/j.jcou.2019.08.025 [DOI] [Google Scholar]
- 29.Xia D, Quan P, Piao H, et al. Preparation of stable nitrendipine nanosuspensions using the precipitation-ultrasonication method for enhancement of dissolution and oral bioavailability. Eur J Pharm Sci. 2010;40(4):325–334. doi: 10.1016/j.ejps.2010.04.006 [DOI] [PubMed] [Google Scholar]
- 30.El-Mahdy MM, Hassan AS, El-Badry M, El-Gindy GE. Performance of curcumin in nanosized carriers niosomes and ethosomes as potential anti-inflammatory delivery system for topical application. Bull Pharm Sci Assiut. 2020;43(1):105–122. doi: 10.21608/bfsa.2020.93599 [DOI] [Google Scholar]
- 31.López-Medina E, López P, Hurtado IC, et al. Effect of Ivermectin on Time to Resolution of Symptoms Among Adults With Mild COVID-19: a Randomized Clinical Trial. JAMA. 2021;325(14):1426–1435. doi: 10.1001/jama.2021.3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vallejos J, Zoni R, Bangher M, et al. Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19) a randomized, double-blind, placebo-controlled trial. BMC Infect Dis. 2021;21(1):635. doi: 10.1186/s12879-021-06348-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmed S, Karim MM, Ross AG, et al. A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. Int J Infect Dis. 2021;103:214–216. doi: 10.1016/j.ijid.2020.11.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kow CS, Merchant HA, Mustafa ZU, Hasan SS. The association between the use of ivermectin and mortality in patients with COVID-19: a meta-analysis. Pharmacol Rep. 2021;73(5):1473–1479. doi: 10.1007/s43440-021-00245-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han AY, Mukdad L, Long JL, Lopez IA. Anosmia in COVID-19: mechanisms and Significance. Chem Senses. 2020;45(6):423–428. doi: 10.1093/chemse/bjaa040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stewart JN, Mounir S, Talbot PJ. Human coronavirus gene expression in the brains of multiple sclerosis patients. Virology. 1992;191(1):502–505. doi: 10.1016/0042-6822(92)90220-J [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arbour N, Day R, Newcombe J, Talbot PJ. Neuroinvasion by human respiratory coronaviruses. J Virol. 2000;74(19):8913–8921. doi: 10.1128/JVI.74.19.8913-8921.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hung EC, Chim SS, Chan PK, et al. Detection of SARS coronavirus RNA in the cerebrospinal fluid of a patient with severe acute respiratory syndrome. Clin Chem. 2003;49(12):2108–2109. doi: 10.1373/clinchem.2003.025437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gu J, Gong E, Zhang B, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202(3):415–424. doi: 10.1084/jem.20050828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.ClinicalTrials.gov. Provided by the U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/results?cond=COVID-19&term=ivermectin&cntry=&state=&city=&dist=. Accessed August 28, 2020.
- 42.Maraie NK, Almajidi YQ. Application of nanoemulsion technology for preparation and evaluation of intranasal mucoadhesive nano-in-situ gel for ondansetron HCl. J Glob Pharm Technol. 2018;10(03):431–442. [Google Scholar]
- 43.Saindane NS, Pagar KP, Vavia PR. Nanosuspension based in situ gelling nasal spray of carvedilol: development, in vitro and in vivo characterization. AAPS PharmSciTech. 2013;14(1):189–199. doi: 10.1208/s12249-012-9896-y [DOI] [PMC free article] [PubMed] [Google Scholar]