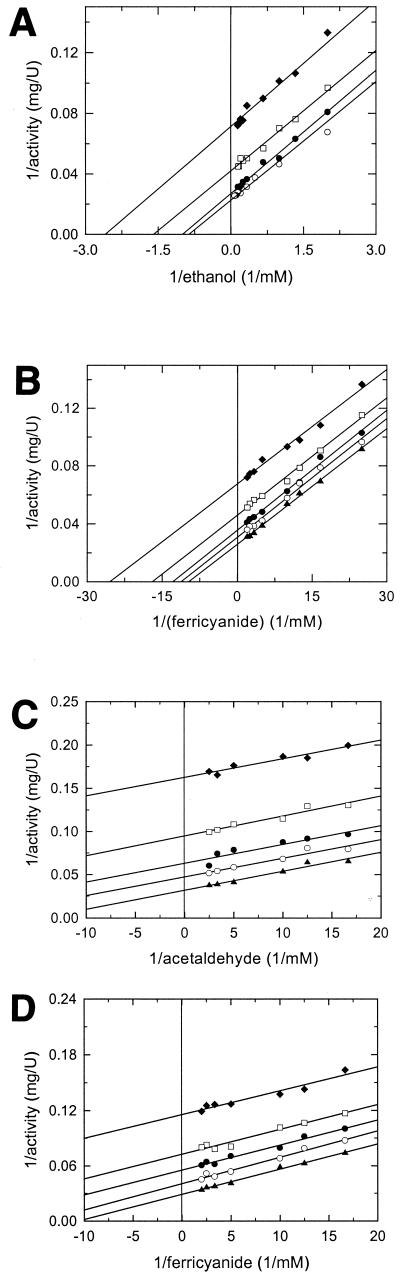
FIG. 2.
Analysis of the reaction mechanism of THFA-DH from R. eutropha strain Bo in the in vitro enzyme assay with ethanol or acetaldehyde as a substrate and ferricyanide as an electron acceptor. The initial rate measurements are depicted as Lineweaver-Burk plots. The activity was determined with 20 mM MOPS-NaOH, pH 8.0, containing 5 mM CaCl2 and 18.8 nM THFA-DH. In case ethanol was used as a substrate, 2 mM semicarbazide was added to the reaction mixture. (A) Initial rates at varying ethanol concentrations, depending on the ferricyanide concentration used: 0.05 (⧫), 0.1 (□), 0.25 (●), and 0.5 (○) mM. (B) Initial rates at varying ferricyanide concentrations, depending on the ethanol concentration used: 0.5 (⧫), 1.0 (□), 2.0 (●), 3.0 (○), and 5.0 (▴) mM. (C) Initial rates at varying acetaldehyde concentrations, depending on the ferricyanide concentration used: 0.02 (⧫), 0.04 (□), 0.06 (●), 0.1 (○), and 0.3 (▴) mM. (D) Initial rates at varying ferricyanide concentrations depending on the acetaldehyde concentration used: 0.02 (⧫), 0.04 (□), 0.06 (●), 0.1 (○), and 0.3 (▴) mM.