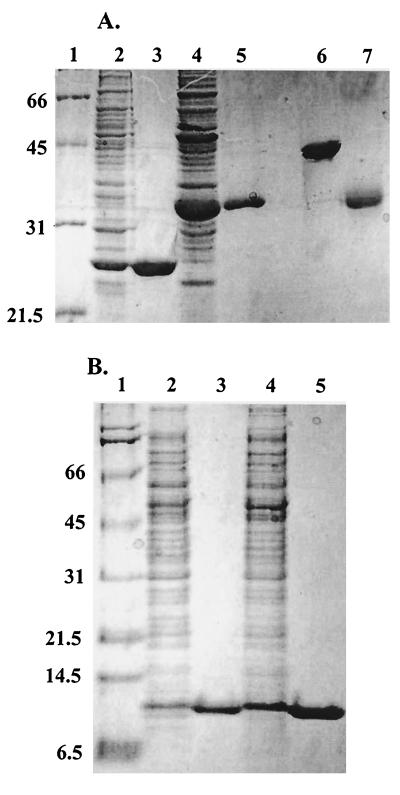
FIG. 1.
SDS-PAGE analysis of purified AhpC, Trx1, Trx2, and TrxR from H. pylori. (A) Crude lysates and recombinant purified AhpC and TrxR were analyzed on the same 12% polyacrylamide gel in reducing sample buffer (except where noted) as follows: lane 1, molecular mass markers (Broad Range Molecular Weight Standards; Bio-Rad); lane 2, crude extracts of E. coli cells transformed with pPROK1/ahpC and induced with 0.4 mM IPTG for at least 3 h at 37°C; lane 3, pure, recombinant AhpC; lane 4, crude extracts of E. coli cells transformed with pPROK1/trxR and induced as described above; lane 5, pure, recombinant TrxR protein; lane 6, pure AhpC in nonreducing sample buffer; lane 7, pure TrxR in nonreducing sample buffer. (B) Crude lysates and recombinant Trx1 and Trx2 were analyzed on a 10% Tris-Tricine gel as follows: lane 1, molecular mass markers; lane 2, crude extracts of E. coli cells transformed with pPROK1/trx1 after induction with IPTG; lane 3, pure, recombinant Trx1; lane 4, crude extracts of E. coli cells transformed with pPROK1/trx2 after induction with IPTG; lane 5, recombinant purified Trx2 after purification. Equivalent protein masses (10 μg) were loaded in all lanes of both gels. Molecular masses (in kilodaltons) are indicated on the left.