Abstract
Chronic hepatitis B (CHB) virus infection is an important threat to global health despite the administration of vaccines and the use of antiviral treatments. In recent years, as the prevalence of obesity and metabolic syndrome has increased, non-alcoholic fatty liver disease (NAFLD) in patients with CHB has become more common. Both diseases can lead to liver fibrosis and even hepatocellular carcinoma, but the risk of dual etiology, outcome, and CHB combined with NAFLD is not fully elucidated. In this review, we assess the overlapping prevalence of NAFLD and CHB, summarize recent studies of clinical and basic research related to potential interactions, and evaluate the progressive changes of treatments for CHB patients with NAFLD. This review increases the understanding of the relationship and mechanisms of interaction between steatosis and hepatitis B virus infection, and it provides new strategies for the future clinical management and treatment of CHB combined with NAFLD.
Keywords: Chronic hepatitis B, Non-alcoholic fatty liver disease, Steatosis, Mechanism
Introduction
Chronic hepatitis B (CHB) virus infection is a well-known threat to global health despite the availability of vaccines and antiviral treatments, and nearly 292 million people (3.9% of the world's population) are living with hepatitis B virus (HBV) infection (defined as being hepatitis B surface antigen [HBsAg]-positive).[1,2] HBV infection results in >820,000 deaths worldwide each year from cirrhosis and hepatocellular carcinoma (HCC).[3]
Non-alcoholic fatty liver disease (NAFLD) has emerged as another common chronic liver disease that affects a quarter of the global population.[4] NAFLD is related to obesity and it includes a series of pathologies such as steatosis, non-alcoholic steatohepatitis (NASH), fibrosis, and cirrhosis. NAFLD also increases the risk of HCC.[5] Global prevalence of NAFLD is 29.8% (95% confidence interval [CI] 28.6–31.1) with the highest prevalence in South America and the lowest in Africa.[6] Our previous meta-analysis included 237 studies (13,044,518 participants) in Asia, and it showed that the overall prevalence of NAFLD was 29.62% (95% CI 28.13–31.15), which was similar to the prevalence trends reported in the western world. The data above indicated that NAFLD is a global disease, regardless of the diagnostic method used.[7] In addition, NAFLD is strongly associated with obesity, insulin resistance, metabolic syndrome, and cardiovascular disease.[8]
As the prevalence of NAFLD increases, the coexistence of NAFLD and HBV infection is also increasing. The overall prevalence of NAFLD in CHB patients is approximately 14% to 70%.[9,10] The existing global epidemiological data on CHB infection combined with NAFLD are mostly from single-center, retrospective cohort studies. The precise prevalence of NAFLD in CHB is widely variable due to different research cohorts that have included different populations and different diagnostic methods for NAFLD, such as ultrasound, transient elastography, or liver biopsy. A recent meta-analysis showed that the pooled prevalence of hepatic steatosis (HS) in CHB was 32.8% (95% CI 28.9−37.0), and it was especially higher in men and obese patients.[11] Because both HBV infection and NAFLD can cause chronic liver injury, exacerbate liver damage, increase the risk of cirrhosis and HCC, and seriously endanger liver health, the relationship between these two diseases has attracted increasing attention.[12,13] However, some studies have shown that the incidence of NAFLD is positively associated with a reduction in HBV serum markers in patients with CHB. We systematically reviewed the research progress related to HBV and NAFLD, trying to unravel the potential relationship between the two diseases, then help to explain the paradox above.
Interaction of CHB and NAFLD
How does NAFLD affect CHB disease progression?
The disappearance of HBsAg and undetectable HBV-DNA in the serum of patients with CHB are important hallmarks of a functional cure.[14] HBsAg seroclearance rarely occurs in the natural history of CHB infection, and prior cohort studies have shown that the annual HBsAg seroclearance rate can range from 0.12% to 2.38%.[15] In addition, even with interferons and antiviral therapy, the incidence of HBsAg serological clearance is still very low. Most of the recent studies have concluded that HS is positively correlated with HBsAg serum clearance and HBV-DNA suppression.
A study by Chu et al[16] in 54 HBsAg carriers with HBsAg seroclearance found that compared with 108 carriers with HBsAg persistence, moderate-to-severe HS may contribute to HBsAg seroclearance in HBsAg carriers. In addition, their further research of 155 patients indicated that HBsAg carriers with a fatty liver on ultrasound and HS could accelerate HBsAg seroclearance by approximately 5 years.[17] Similarly, our retrospective cohort study of 6786 adult patients with CHB found that compared to patients with non-fatty liver CHB, patients with fatty liver CHB had a higher HBsAg seroclearance rate.[18] We previously found that CHB patients with NAFLD were significantly more likely to be hepatitis B e antigen (HBeAg) negative (74.3% vs. 62.8%) compared with CHB patients without NAFLD.[19] Tai et al[20] demonstrated a significantly higher incidence of HBsAg clearance in HBeAg-seronegative CHB patients with HS than in those without. They employed Cox regression analysis to further identify HS as an independent predictor of spontaneous HBsAg seroclearance in CHB patients, including HBeAg-seropositive and -seronegative (hazard ratio [HR] = 1.222).[21] Extreme obesity and central obesity were associated with a low prevalence of high HBV viral load in HBeAg-seropositive patients, especially in men. However, hypertriglyceridemia was associated with a low prevalence of high viral load in HBeAg-negative patients, both women and men.[22]. Increased HS was independently associated with decreased serum HBV DNA levels, suggesting a potential negative impact on viral replication.[23] Moreover, Wang et al[24] performed a study on 3212 patients with HBV infection and showed that the severity of HS in patients was negatively correlated with the expression of intrahepatic HBsAg and hepatitis B core antigen (HBcAg). It is important to note that the positive correlation between HS and HBeAg/HBsAg serologic clearance or HBV-DNA suppression should be viewed dialectically, and we cannot assume that NAFLD is beneficial in CHB patients. Despite HBV seromarkers being reduced in patients with CHB and NAFLD, the clinical outcomes of these patients may be worse.
While there exists growing evidence that NAFLD may provide some benefit in reducing disease progression in CHB patients, there are some studies that have suggested otherwise. Jin et al[25] retrospectively analyzed 720 CHB patients in 3 years and found that HS was not significantly associated with HBsAg seroclearance, fibrosis progression, or HCC development. However, their study may be influenced by its retrospective nature with a relatively short follow-up duration.
There have also been some studies that verified the negative association between NAFLD and HBV in cellular and animal models. Zhang et al[26] established mice with chronic HBV genotype B infection using a microinjection of oocytes, and HS was induced by feeding them high-fat diets. The research found that the model mice suffering from both CHB and NAFLD had reduced HBV viral replication.[26] Similar results were obtained by Hu et al[27] in an HBV-immunocompetent mouse model where NASH inhibited HBV-DNA replication and reduced the expression levels of HBeAg and HBsAg. Another study verified this result from both cell and mouse models, and it proposed that saturated fatty acids (SFAs) can inhibit HBV replication in CHB combined with NAFLD by activating the toll-like receptor 4 (TLR4) signaling pathway.[28]
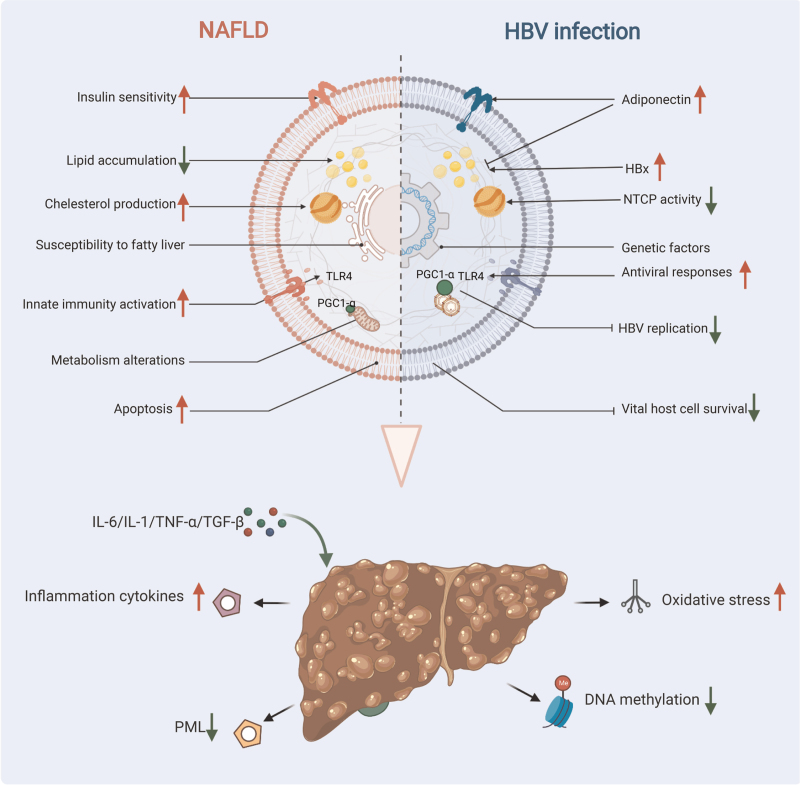
The possible mechanistic role of NAFLD in HBV infection remains unclear, and there are several speculations. As mentioned above, TLR4 is a cell surface receptor that is crucial for the activation of innate immune responses. HBV infection restricts interferon production and interferon-stimulated gene activation by the inhibition of the myeloid differentiation primary response 88 (MyD88) signaling pathway, leading to innate immunodeficiency and chronic HBV infection.[29,30] Our previous study indicated that TLR4 induced MyD88/nuclear factor-kappa B downstream activation in B cells triggered by HBV.[31] Zhang et al[28] found that SFAs served as a potential ligand for TLR4 and activated the TLR4 signaling pathway, and this might be involved in accelerating the mechanism of inhibiting HBV replication in CHB with NAFLD. Some research has shown that metabolic factors in NAFLD may influence HBV replication. For example, Shlomai et al[32] found that fasting stimulated HBV DNA replication in vivo, and this was primarily due to an increase in the peroxisome proliferator-activated receptor-γ coactivator (PGC)-1α activity. PGC-1α is a transcription factor that plays an important role in gluconeogenesis,[32] and simultaneously, PGC-1α is lowly expressed in NAFLD.[33] Therefore, the metabolic alterations induced by NAFLD may inhibit HBV replication by suppressing the expression of PGC-1α. However, apoptosis in HBV-infected cells has been reported to inhibit viral replication in these cells.[34] In addition, increased Fas-mediated apoptosis has been observed in liver samples from patients with NAFLD.[35,36] It is hypothesized that NAFLD may accelerate HBsAg and HBV-DNA clearance by increasing apoptosis in HBV-infected cells. We summarize the possible pathways and mechanisms as Figure 1.
Figure 1.
Potential mechanisms of interaction between NAFLD and CHB and clinical outcomes. Metabolic alterations in NAFLD patients may enhance antiviral responses through activation of innate immunity, such as by activating the TLR4 signaling pathway or suppressing the expression of PGC-1α. NAFLD could also accelerate HBsAg and HBV-DNA clearance by increasing apoptosis in HBV-infected cells. Hepatic steatosis in CHB patients is associated with host metabolic factors, a reduced risk of hyperlipidemia has been observed in patients with CHB. In addition, the higher serum adiponectin level could account for the lower prevalence of steatosis in HBV-infected subjects. Conversely, the overexpression of HBx and the genetic susceptibility to fatty liver in CHB patients induce hepatic lipid accumulation. There are many potential mechanisms for clinical outcomes in NAFLD combined with CHB. Inflammatory cytokines (eg, IL-1, IL-6, TNF-α, TGFβ) released from damaged hepatocytes in NASH can lead to activation and proliferation of hepatic stellate cells. NAFLD-related fat deposition and oxidative stress may create a pro-fibrotic and pro-carcinogenic milieu within the liver. In addition, the PML deficiency-mediated abnormal lipid metabolism induced by HBsAg and the lower levels of global DNA methylation in patients with concurrent NAFLD and CHB could both accelerate the development of cirrhosis and HCC. CHB: Chronic hepatitis B; HBsAg: Hepatitis B surface antigen; HBV: hepatitis B virus; HBx: Hepatitis B protein X; NAFLD: Non-alcoholic fatty liver disease; NASH: Non-alcoholic steatohepatitis; PGC-1α: Peroxisome proliferator-activated receptor-γ coactivator-1α; PML: Promyelocyticleukemia protein; TLR4: Toll-like receptor 4; NTCP: Sodium taurocholate cotransporting polypeptide; IL-1: Interleukin-1; IL-6: Interleukin-6; TNF-α: Tumor necrosis factor-α; TGFβ: Transforming growth factor β.
Do CHB patients have a decreased risk of NAFLD onset?
It is now well established that hepatitis C virus (HCV) infection affects changes in insulin resistance and lipid metabolism, leading to HS and more severe inflammation in patients with chronic hepatitis C (CHC). HS is also a common histological feature of CHC. Unlike HCV, studies of HS and CHB are limited. There is currently no direct evidence that HBV increases the risk of steatosis. A meta-analysis revealed HS in HBV seems to be as frequent as in the general population, and lower than in HCV-infected patients. In addition, there was a strong negative correlation between viral load and HS, which may indicate a protective effect of the virus on HS.[10]
The results of recent studies have indicated a negative association with a possible risk for steatosis in CHB [Table 1]. In a large-scale cohort study of 83,339 participants, the incidence of steatosis was significantly lower in CHB patients than in the controls. Even after adjusting for possible confounders and metabolic factors, there was still a significant negative correlation between HBV infection and NAFLD.[37] A cross-sectional study using proton-magnetic resonance spectroscopy, a highly reliable steatosis assay, to determine fatty liver status found that chronic HBV infection was significantly associated with a lower risk of fatty liver.[38] Another large case–control study in China confirmed this conclusion.[39] The results of four additional studies indicated a negative correlation between steatosis and CHB.[40–43]
Table 1.
Publications on the potential role of CHB in NAFLD onset.
| References | Year of publication | Number of patients | Explored association between CHB and NAFLD onset | OR, RR, or HR |
| Wong et al[38] | 2012 | 1030 | HBsAg seropositivity and fatty liver | 0.42 (0.20–0.88) |
| Cheng et al[44] | 2013 | 33,439 | HBsAg seropositivity and fatty liver | 0.70 (0.64–0.76) |
| Huang et al[40] | 2020 | 14,452 | HBsAg seropositivity and NAFLD | 0.72 (0.61–0.85) |
| Wang et al[41] | 2018 | 1882 | HBsAg seropositivity and NAFLD | 0.57 (0.34–0.98) |
| Lin et al[42] | 2021 | 4734 | HBsAg seropositivity and NAFLD | 0.83 (0.78–0.89) |
| Lv et al[43] | 2021 | 16,451 | HBsAg seropositivity and hypercholesterolemia | 0.62 (0.58–0.66) |
| Zhong et al[39] | 2018 | 2988 | HBsAg seropositivity and NAFLD | 0.64 (0.42–0.95) |
| Yun et al[47] | 2009 | 86 | HBsAg seropositivity and hypercholesterolemia | 1.19 (1.17–2.83) |
| Joo et al[37] | 2017 | 83,339 | HBsAg seropositivity and NAFLD | 0.83 (0.73–0.94) |
| Zhu et al[45] | 2019 | 4429 | HBV DNA levels and NAFLD | 0.37 (0.14–0.98) |
CHB: Chronic hepatitis B; HBsAg: Hepatitis B surface antigen; HBV: Hepatitis B virus; HR: Hazard ratio; NAFLD: Non-alcoholic fatty liver disease; RR: Relative risk; OR: Odds ratio.
To date, various studies seem to indicate that HBV may have a protective effect on the development of the fatty liver. In a large-scale study in Taiwan (China) by Cheng et al[44] that recruited a total of 33,439 subjects, they found an inverse relationship between HBV infection and NAFLD in subjects older than 50 years and no significant association between HBV infection and fatty liver in subjects younger than 50 years. Zhu et al[45] found no association between viral factors and the NAFLD incidence rate in 2393 CHB patients who had no history of alcohol intake. Moreover, steatosis was observed in 62% of patients according to the research of Viganò et al[46], and it was independently associated with body mass index (BMI) and hyperglycemia, but not with virologic factors such as HBeAg status or HBV-DNA load. The authors speculated that metabolic alterations are the leading cause of steatosis in CHB.[46] In addition, three case-control studies have shown that HBeAg status and HBV DNA are not related to liver steatosis in patients with CHB.[47–49] Enomoto et al[50] obtained a similar result in the histology of patients with high HBV-DNA levels. Wang et al[41] found that HBV infection is not associated with fatty liver disease occurrence, and they verified the results in vitro. Conversely, it was revealed that HBV may play a role in the development of NAFLD by increasing the production of mitochondrial reactive oxygen species in vitro.[41]
Hu et al[27] found that in an HBV-immunocompetent mouse model, NASH inhibited HBV replication, while virus replication did not alter lipid metabolism. Similarly, Zhang et al[26] found that in HBV genotype B transgenic mice with combined NAFLD, HBV viral factors were reduced compared to the controls, without reducing the metabolic and histologic features. Both mouse models had their shortcomings. HBV transgenic mice are immune-tolerant and cannot be spontaneously cleared as their genomes are integrated into the mouse genome. HBV-immunocompetent mice with a hydrodynamic injection can effectively transfer exogenous genes into liver cells; however, viremia peaks after 6 days and rapidly declines thereafter. Although these animal models differ from HBV infection in humans, the studies still play important roles in the study of HBV combined with NAFLD.
Some studies have pointed out that HS in CHB patients is associated with host metabolic factors. Hyperlipidemia is an important risk factor for NAFLD; however, a reduced risk of hyperlipidemia has been observed in patients with CHB. A cross-sectional study of 7695 Taiwanese adults demonstrated a lower risk of hypercholesterolemia, hypertriglyceridemia, and high low-density lipoprotein cholesterol levels in HBV-infected individuals.[51] In addition, two large community-based cohort studies found a negative association between chronic HBV infection and serum lipid levels, such as triglycerides (TG) and cholesterol.[52,53] In another retrospective cohort of 122 patients with CHB, a negative association between serum HBV DNA levels and TG levels was also found.[54]
Furthermore, HBV infection may alter hepatic cholesterol metabolism. Some studies have shown that the pre-S1 domain of the HBV envelope can hinder sodium taurocholate cotransporting polypeptide (NTCP) mediated bile acid uptake and lead to compensatory cholesterol production and uptake. In addition, adiponectin improves hepatic insulin sensitivity and decreases lipid accumulation in macrophages.[55,56] CHB patients have been reported to have higher serum adiponectin levels, and this could account for the low prevalence of steatosis in HBV-infected subjects.[57] In addition, Hajjou et al[58] identified a significant upregulation of lipid biosynthesis gene expression in the liver of HBV transgenic mice, including adenosine 5′-triphosphate citrate lyase, fatty acid synthase, sterol regulatory element-binding factor 2, and retinol-binding protein 1. Yilmaz et al[59] found that in patients infected with HBV, HS was primarily associated with metabolic factors, such as obesity, insulin resistance, and dyslipidemia, rather than viral factors.
Hepatitis B protein X (HBx), one of the four HBV proteins, has an important role in HBV infection. Previous studies have confirmed that the overexpression of HBx induces hepatic lipid accumulation in HepG2-HBx stable cells and in HBx-transgenic mice. Therefore, HBx is a risk factor for steatosis.[41] Kang et al[60] showed that hepatic lipid accumulation may be caused by the inhibition of apolipoprotein B secretion by HBx protein, while Kim et al[61] suggested that increased HBx expression mediated by sterol regulatory element-binding protein 1 and peroxisome proliferator-activated receptor γ (PPAR-γ) also increases hepatic lipid accumulation. Subsequently, HBx has been reported to upregulate fatty acid-binding protein 1 to promote hepatic lipid accumulation in the development of steatosis in HBV-induced cells.[62] In addition, in HBx-transfected hepatocytes, HBx stimulated the expression and transcriptional activation of CCAAT/enhancer-binding protein α (C/EBPα) and PPAR-γ, both regulators of C/EBPα adipocyte differentiation.[61]
The evidence suggested the possibility of genetic susceptibility to fatty liver in CHB. Single-nucleotide polymorphisms (SNPs) of patatin-like phospholipase domain-containing protein 3 (PNPLA3), and transmembrane 6 superfamily member 2 (TM6SF2) are two important genetic determinants of NAFLD.[63,64] In cohort studies of biopsy-proven CHB patients with or without HS, several SNPs of PNPLA3 were independently associated with steatosis and inflammation. In addition, when compared to the TT genotype, the C allele at PNPLA3 rs1010023 conferred a higher risk of HS in CHB patients (OR = 1.768, 95% CI: 1.027−3.105; P = 0.045).[65] The T allele of rs58542926 in TM6SF2 has been associated with altered lipids and HS in CHB patients.[66] The genetic and metabolic changes related to HBV infection at the cellular level could help explain the clinical outcomes, but phenotypic differences related to NAFLD at the individual level require further study. We summarize the possible pathways and mechanisms as Figure 1.
Progression and Outcomes of CHB with NAFLD
In NAFLD patients, fibrosis is the feature most closely associated with long-term adverse events compared to other histological features. NASH, a severe form of NAFLD, has a rapid progression in fibrosis, and it is the major cause of liver fibrosis, cirrhosis, and HCC in advanced NAFLD.[67] In developed countries, NAFLD could account for >30% of HCC cases.[5] As we know, CHB infection is an important cause of HCC formation, and previously, >70% of the HCC incidence was attributed to chronic viral hepatitis.[68] Both diseases are capable of independently increasing the risk of HCC development. Today, patients with CHB have an increased incidence of combined HS, but there is no conclusive evidence linking HS and liver fibrosis, cirrhosis, and HCC in patients with CHB infection. Table 2 is a summary of the recent studies.
Table 2.
Publications on the progression and outcomes of CHB with NAFLD.
| References | Year of publication | Number of patients | HS and fibrosis (surrogate/method) | HBsAg seroclearance | Fibrosis progression | Cirrhosis progression | HCC development |
| Li et al[18] | 2021 | 6786 | Ultrasonography or CT | More in FL | Not reported | Less in FL | Less in FL |
| Chang et al[25] | 2021 | 720 | Transient elastography | No difference | No difference | Not reported | No difference |
| Cheng et al[69] | 2016 | 1466 | Transient elastography | Not reported | No difference | No difference | No difference |
| Lee et al[13] | 2019 | 321 | Liver biopsy | Not reported | Not reported | Not reported | No difference |
| Lim et al[73] | 2020 | 289 | Liver biopsy | Not reported | Not reported | Not reported | No difference |
| Chen et al[70] | 2017 | 162 | Liver biopsy | Not reported | More in HS | No difference | Not reported |
| Mandana et al[74] | 2021 | 420 | Liver biopsy | Not reported | More in steatohepatitis | No difference | Not reported |
| Wong et al[78] | 2020 | 614 | Transient elastography | Not reported | More in HS | Not reported | No difference |
| Chu et al[17] | 2013 | 155 | Ultrasonography | More in HS | Not reported | More in HS | Not reported |
| Mak et al[74] | 2020 | 330 | Transient elastography | More in HS | More in HS | Not reported | Not reported |
| Charatcharoenwitthaya et al[76] | 2017 | 256 | Liver biopsy | Not reported | More in steatohepatitis | Not reported | Not reported |
| Choi et al[9] | 2020 | 1089 | Liver biopsy | Not reported | More in NASH | Not reported | More in NASH |
| van Kleef et al[79] | 2021 | 1076 | Liver biopsy | Not reported | Not reported | Not reported | More in MAFLD |
| Peleg et al[81] | 2019 | 524 | Liver biopsy and ultrasonographic | Not reported | More in liver steatosis | Not reported | More in liver steatosis |
| Kim et al[80] | 2021 | 48,335 | FLI and HSI | Not reported | Not reported | Not reported | More in HS |
| Chan et al[12] | 2017 | 270 | Liver biopsy | Not reported | Not reported | More in FL | More in FL |
| Karacaer et al[72] | 2016 | 254 | Ultrasonography | Not reported | More in HS | Not reported | Not reported |
CHB: Chronic hepatitis B; CT: Computed tomography; FL: Fatty liver; FLI: Fatty liver index; HBsAg: Hepatitis B surface antigen; HS: Hepatic steatosis; HIS: Hepatic steatosis index; MAFLD: metabolic associated fatty liver disease; NAFLD: Non-alcoholic fatty liver disease; NASH: Non-alcoholic steatohepatitis.
In Hong Kong (China), Cheng et al[69] prospectively recruited 1466 patients with CHB from 2006 to 2008. They found that metabolic syndrome increased the risk of cardiovascular events, such as acute coronary syndrome, and cerebral vascular events, but not cirrhotic complications, HCC, and/or liver-related mortality.[69] Similarly, our previous retrospective cohort study reported 6786 adult Asian participants with CHB from 1991 to 2016. During the mean follow-up of 131.88 ± 77.28 months, 564 patients developed cirrhosis, 281 patients developed HCC, and 478 patients had HBsAg seroclearance. Importantly, we found that a combined fatty liver did not increase the occurrence of liver cirrhosis and HCC in the CHB patients.[19] It has also been shown that significant or advanced liver fibrosis is associated with the grade of necroinflammation and host factors but not with HS in CHB patients.[70,71] In addition, several other studies have suggested that HS is not a risk factor for the probability of fibrosis progression or HCC development in CHB patients.[13,25,71–73]
However, most studies have confirmed that obesity, diabetes, and MetS are independent risk factors for fibrosis, cirrhosis, and HCC in CHB patients, suggesting a synergistic effect of metabolic factors and CHB on HCC pathogenesis. A recent cohort study from North America included 420 CHB cases who underwent liver biopsies. The study found that the combination was associated with advanced fibrosis and higher biochemical measures of hepatic inflammation over time.[74] Similar results were obtained in two Hong Kong studies.[12,75] In addition, Charatcharoenwitthaya et al[76] demonstrated that steatohepatitis was related to the severity of liver fibrosis, and Mak et al[77] showed that the rate of liver fibrosis progression in patients with persistent severe steatosis was higher than in those without steatosis in CHB. Moreover, Chen et al[70] found that the 5-year cumulative incidence of cirrhosis was 4.17% in patients without HS and 5.19% in patients with HS, and advanced fibrosis may be a risk factor for progression to cirrhosis in NAFLD. Furthermore, HS may promote the progression of HCC.[70] In particular, Chan et al[12] found that the incidence of HCC was significantly higher in patients with steatosis >5% than in those with steatosis <5%, and that combined fatty liver was an independent risk factor for the development of HBV-related HCC, with an associated 7.3-fold increased risk.
A longitudinal cohort study from Wong et al[78] found that the presence of HS was independently associated with advanced fibrosis. However, HS was not associated with any adverse outcomes in CHB patients. These researchers indicated that HS does not predict adverse outcomes in CHB patients, unlike advanced fibrosis.[78] Conversely, a large combined tertiary center cohort that included 1089 patients who underwent liver biopsies between 1985 and 2016 at one of the two tertiary centers showed that combined severe HS was closely associated with the development of liver fibrosis in CHB patients. The combination of NASH also increases the risk of liver-related events, including decompensated cirrhosis, HCC, and even death, in CHB patients.[9] Similar results were obtained in a recent study by van Kleef et al[79]. Additionally, a study by Kim et al[80] reported that CHB patients with liver steatosis had a higher risk of all-cause mortality and cancer development compared to patients without liver steatosis. Another retrospective study included 524 treatment-naïve patients with CHB with a mean follow-up of 6 years. It found that CHB patients with liver steatosis had an increased risk of all-cause mortality and cancer development compared to CHB patients without liver steatosis, regardless of their baseline HBV viral load.[81] All of these results indicated that in addition to viral suppression, screening for and managing metabolic abnormalities is important to prevent disease progression in HBV.
The mechanism of NAFLD in CHB-related fibrosis, cirrhosis, and HCC remains unclear, possible pathways and mechanisms are shown in Figure 1. It is speculated that NAFLD-mediated inflammatory injury and fibrogenesis in HBV infection are responsible.[74] It is well known that danger-associated molecular patterns released from damaged hepatocytes in NASH as well as inflammatory cytokines can lead to activation and proliferation of hepatic stellate cells, which can exacerbate fibrosis or eventually cirrhosis.[82,83] NAFLD-related accumulated fat deposition, oxidative stress, and a low-grade inflammatory response may create a pro-fibrotic and pro-carcinogenic milieu within the liver and further accelerate the development of CHB-related cirrhosis and HCC. In addition, a recent study found that the knockdown of the promyelocytic leukemia protein (PML; a tumor suppressor involved in genome maintenance and fatty acid oxidation) in HBsAg-transgenic mice predisposed them to obesity and drove early-steatosis-specific liver tumorigenesis. This demonstrated that PML deficiency-mediated abnormal lipid metabolism induced by HBsAg appears to be a hallmark of chronic HBV-associated pathogenesis leading to steatosis-associated hepatocarcinogenesis.[84]
DNA methylation is one of the most common epigenetic modifications, and a loss of global DNA methylation in sequences that are normally methylated can lead to chromosomal abnormalities and ultimately disease development.[85,86] A recent study found that patients with concurrent NAFLD and CHB exhibited lower levels of global DNA methylation than patients with NAFLD alone. The concurrence of CHB and liver fibrosis in NAFLD patients was associated with a decrease in the global DNA methylation levels.[87] NAFLD-related cumulative fat deposition, oxidative stress, and inflammatory responses may produce a pro-fibrotic and pro-cancer environment in the liver and further accelerate the development of cirrhosis and HCC. However, the disease progression is influenced by various confounding factors, and the interaction between HBV and metabolic homeostasis in the progression of liver disease requires further study.
Effect of Antiviral Treatment on NAFLD
Antiviral therapy is commonly used in preventing disease progression and the incidence of HCC in CHB patients. However, the data regarding the implication of HS on antiviral therapy for CHB have been conflicting. A retrospective single-centered cohort study in 2020 found that the presence of NAFLD and metabolic features associated with NAFLD did not negatively affect the complete virologic suppression or biochemical response to CHB treatment outcomes during a follow-up of up to 60 months. In addition, they recommended that CHB patients with NAFLD should not require any special considerations while choosing CHB treatment.[19] Similar results were observed in three other studies in CHB patients treated with entecavir (ETV) or tenofovir disoproxil fumarate (TDF).[88–91] In addition, Chen et al[92] found that HS had no significant impact on the HBeAg seroclearance in HBeAg-positive CHB patients with nucleo(t)ide analog (NA) treatment. Moreover, a retrospective multicenter cohort study that included a total of 145 cases with chronic HBV infection from three different medical centers indicated that NAFLD did not affect the virological response at 6 and 12 weeks of ETV or TDF treatment. In addition, the presence of steatohepatitis was found to predict a more favorable response to treatment at 6 months in the TDF arm than in patients without.[93]
However, it has also been demonstrated that the efficacy of antiviral therapy is worse in CHB patients with combined HS. Liang et al[94] found that HS can reduce the efficacy of PEG-IFNalpha-2a in the treatment of CHB patients, and its mechanism may be related to different HBcAg expression patterns in liver tissue. Jin et al[95] found that HS was a significant independent factor associated with ETV treatment failure in a prospective study of 267 Chinese patients with CHB infection. Concurrently, they indicated this was due to lipid accumulation and the reduced activity of cytochromes in HS on drug metabolism.[95] In addition, a meta-analysis that included eight prospective cohort studies showed that the biochemical and virological responses at both 48 and 96 weeks were significantly lower in patients with CHB with HS as compared to that in patients with only CHB. HS lowers the efficacy of antiviral treatment in patients with CHB, especially when HS is diagnosed on ultrasound findings and treated with NAs.[96] Recently, a study reported that the NA-treated group increased body fat mass and visceral fat area in CHB patients.[97]
Some studies have indicated that NA therapy might influence HS, and NA therapy may contribute to body fat accumulation in CHB patients.[98] An in vitro study reported that TDF modulated lipid metabolism by upregulating hepatic CD36 via activating PPAR-α.[99] The high expression of hepatic CD36 improved HS and insulin resistance by reducing hepatic lipids, and this may explain the findings. In a study of patients with CHB, switching to tenofovir alafenamide improved metabolic dysfunction, reduced the serum alanine aminotransferase (ALT) levels, and promoted ALT normalization in patients, despite significant increases in body weight and BMI.[100] Although the impact of antiviral therapy on HS in these patients remains debatable, the onset or progression of NAFLD during antiviral therapy should still be monitored in case of underlying negative impacts.
In contrast, there are fewer studies that have examined the effect of fatty liver treatment on CHB progression. A retrospective cohort study in Korea showed that of 13,063 patients with CHB, 980 (7.5%) were statin users and 12,083 (92.5%) were statin-naïve. The annual liver cancer mortality was significantly lower among statin users than non-users. A multivariable analysis after adjusting for demographic and metabolic factors showed consistent results.[101] This study had some limitations. It did not include detailed data on the serum HBV DNA levels or cirrhosis status. In addition, this study did not consider the effect of antiviral therapy on liver cancer mortality. In addition, the study population consisted exclusively of Korean populations, which limits the generalizability of the findings. We also require more studies regarding the effects of NAFLD treatments, such as medications and bariatric surgery, on the progression of HBV infection and antiviral efficacy.
Potential Cell and Animal Models for Researching CHB and NAFLD
In vitro cell models supporting HBV replication and infection are important tools for basic studies of the HBV life cycle, and these models play essential roles in the identification of novel anti-HBV targets and efficacy evaluations of drug candidates while helping to better investigate the mechanisms of HBV combined with NAFLD.
Human hepatocytes are specific hosts for HBV. For quite some time, primary human hepatocytes (PHH) were the only research model supporting HBV infection in vitro.[102] However, due to the limited source of human primary hepatocytes, the demanding in vitro culture techniques, and the inability to continuously expand have limited their application in HBV research.[103] Due to the availability of PHH and donor problems, HepaRG cells have been investigated as an alternative source. The bipotential progenitor-like HepaRG cell line was isolated from an HCV-induced hepatocarcinoma and can be induced to differentiate as hepatocytes or cholangiocytes in vitro.[104] Human hepatoma cell lines that replicate HBV after transfection or the stable integration of HBV genomes have been widely used for the analysis of HBV replication and viral-host interactions, and HepG2.2.15 is the most commonly used of these.[105,106] NTCP is a multiple transmembrane bile acid transporter protein specifically expressed on the surface of hepatocyte membranes that binds specifically to the pre-S1 region of the HBV viral outer membrane protein and is a functional receptor for HBV that is absent from expression in HCC cell lines. Therefore, the commonly used HCC cell lines, such as HepG2 and Huh7 are resistant to HBV infection, but exogenous expression of NTCP in them can restore their HBV susceptibility.[107] It has been widely used for the study of basic biological problems of HBV and also has provided tools for the development of early antiviral drugs. To investigate the mutual effects and mechanisms of CHB patients with combined fatty liver in vitro, palmitic acid and oleic acid are the most common free fatty acids that can induce steatosis in HBV cell models.[28]
In addition to cellular models, animal models are crucial to imitate NAFLD and HBV infection simultaneously. HBV can only infect humans and chimpanzees, but the use of chimpanzees in HBV research is strongly restricted.[108] To date, the tree shrew (Tupaia belangeri) is the only non-primate animal found to be susceptible to HBV infection. Primary Tupaia hepatocytes are readily available compared with PHH, and they have been used in HBV research for many years.[109]
Humanized chimeric mouse
The first human liver chimeric mouse model was developed in immunodeficient (Rag2−/−, server combined immune-deficiency [SCID], SCID/beige) mice using the urokinase-type plasminogen activator (uPA) transgene. The transplantation of human hepatocytes into uPA-SCID mice results in a liver-humanized model with a high human hepatocyte reconstitution rate that is supportive of HBV and HCV infection.[110,111] These models can simulate the natural history of HBV infection in vivo as well as the innate and adaptive immune responses against HBV.
HBV transgenic mouse model
It has long been reported in the literature that HBV transgenic mice that selectively express HBV proteins have been used to study topics related to HBV infection. In 1995, a full HBV genome transgenic mouse model was established, producing infectious HBV virions morphologically identical to human-derived virions.[112] Although HBV is immunotolerant and cannot be spontaneously cleared due to its genomic integration into the mouse genome, this model has made an important contribution to elucidating the pathogenesis of HBV infection.
HBV transfected mouse model
Adeno-associated viral vectors containing the HBV genome efficiently transduce hepatocytes in immunocompetent mice. HBV replication can last for 3 months after intravenous injection of adenoviral vectors containing HBV.[113] Rapid injection of large amounts of liquid containing naked DNA into mice by the tail vein, called hydrodynamic injection, which can effectively transfer exogenous genes into hepatocytes. Hydrodynamic injection was found to successfully deliver replication-competent HBV genome into the liver of immunocompetent mice, with viremia peaking after 6 days and declining rapidly thereafter.[114]
Mouse models of NAFLD
Animal models of NAFLD are primarily based on various types of diet, such as a HFD, a high glucose, sucrose, fructose, methionine, and choline-deficient (MCD) diet, a choline-deficient L-amino acid-defined diet, a high-cholesterol diet, and a cholesterol and cholate diet. Even in the genetic animal models of NASH, diet is used as a means of a secondary trigger for disease progression.[115] A HFD composed of 71% fat, 11% carbohydrates, and 18% proteins fed to mice for 16 weeks is known to result in the development of insulin resistance with marked panlobular steatosis, inflammation, and induce fibrosis.[116] The model displays NAFLD features similar to human NAFLD, but the pathological outcome is not as severe.[115] MCD is a commonly used diet that produces the most severe phenotype of NASH in the shortest amount of time. This diet has high sucrose (40%) and 10% fat, but it is deficient in methionine and choline, and it is known to quickly induce measurable hallmarks of NAFLD, such as HS in mice and rats in 2 to 4 weeks, and this progresses to inflammation and fibrosis shortly thereafter.[117,118]
These HBV mouse models combined with well-established NAFLD mice models, such as HFD or MCD, promise to serve as applicable alternative models for exploring the interaction of HBV infection and NAFLD in vivo.
Conclusions
Both NAFLD and CHB are chronic liver diseases that inevitably threaten the health of patients by disrupting liver function and exacerbating end-stage cirrhosis and HCC. Although there are abundant results that have demonstrated serum clearance of hepatitis B in patients with HS, the prevalence of NAFLD may still be lower in CHB patients. There remains a knowledge gap on how this happens. The outcome of treatment with oral antivirals or interferons appears to be unaffected by HS. However, viral factors appear to influence necroinflammation and fibrosis in the liver rather than HS. As the mechanisms of interactions between steatosis and HBV infection become clearer, future studies will provide new strategies for the clinical management and treatment of CHB combined with NAFLD.
Funding
This study was supported by grants from the National Natural Science Fund (Nos. 81970545, 82170609), the Natural Science Foundation of Shandong Province (Major Project) (No. ZR2020KH006), the Ji’nan Science and Technology Development Project (No. 202019079), and the Nanjing Medical Science and Technique Development Foundation (No. YKK20058).
Conflicts of interest
None.
Footnotes
How to cite this article: Tong X, Song Y, Yin S, Wang J, Huang R, Wu C, Shi J, Li J. Clinical impact and mechanisms of hepatitis B virus infection concurrent with non-alcoholic fatty liver disease. Chin Med J 2022;135:1653–1663. doi: 10.1097/CM9.0000000000002310
References
- 1.Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol 2018; 3:383–403. doi: 10.1016/S2468-1253(18)30056-6. [DOI] [PubMed] [Google Scholar]
- 2.Shi Y, Zheng M. Hepatitis B virus persistence and reactivation. BMJ 2020; 370:m2200.doi: 10.1136/bmj.m2200. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Hepatitis B. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b. [Accessed 30 May, 2022.] [Google Scholar]
- 4.Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet 2021; 397:2212–2224. doi: 10.1016/S0140-6736(20)32511-3. [DOI] [PubMed] [Google Scholar]
- 5.Sanyal AJ, Van Natta ML, Clark J, Neuschwander-Tetri BA, Diehl A, Dasarathy S, et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med 2021; 385:1559–1569. doi: 10.1056/NEJMoa2029349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016; 64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Zou B, Yeo YH, Feng Y, Xie X, Lee DH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2019; 4:389–398. doi: 10.1016/S2468-1253(19)30039-1. [DOI] [PubMed] [Google Scholar]
- 8.Samuel VT, Shulman GI. Nonalcoholic fatty liver disease, insulin resistance, and ceramides. N Engl J Med 2019; 381:1866–1869. doi: 10.1056/NEJMcibr1910023. [DOI] [PubMed] [Google Scholar]
- 9.Choi HSJ, Brouwer WP, Zanjir WMR, de Man RA, Feld JJ, Hansen BE, et al. Nonalcoholic steatohepatitis is associated with liver-related outcomes and all-cause mortality in chronic hepatitis B. Hepatology 2020; 71:539–548. doi: 10.1002/hep.30857. [DOI] [PubMed] [Google Scholar]
- 10.Machado MV, Oliveira AG, Cortez-Pinto H. Hepatic steatosis in hepatitis B virus infected patients: meta-analysis of risk factors and comparison with hepatitis C infected patients. J Gastroenterol Hepatol 2011; 26:1361–1367. doi: 10.1111/j.1440-1746.2011.06801.x. [DOI] [PubMed] [Google Scholar]
- 11.Zheng Q, Zou B, Wu Y, Yeo Y, Wu H, Stave CD, et al. Systematic review with meta-analysis: prevalence of hepatic steatosis, fibrosis and associated factors in chronic hepatitis B. Aliment Pharmacol Ther 2021; 54:1100–1109. doi: 10.1111/apt.16595. [DOI] [PubMed] [Google Scholar]
- 12.Chan AW, Wong GL, Chan HY, Tong JH, Yu YH, Choi PC, et al. Concurrent fatty liver increases risk of hepatocellular carcinoma among patients with chronic hepatitis B. J Gastroenterol Hepatol 2017; 32:667–676. doi: 10.1111/jgh.13536. [DOI] [PubMed] [Google Scholar]
- 13.Lee YB, Ha Y, Chon YE, Kim MN, Lee JH, Park H, et al. Association between hepatic steatosis and the development of hepatocellular carcinoma in patients with chronic hepatitis B. Clin Mol Hepatol 2019; 25:52–64. doi: 10.3350/cmh.2018.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lok AS, Zoulim F, Dusheiko G, Ghany MG. Hepatitis B cure: from discovery to regulatory approval. Hepatology 2017; 66:1296–1313. doi: 10.1002/hep.29323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tout I, Loureiro D, Mansouri A, Soumelis V, Boyer N, Asselah T. Hepatitis B surface antigen seroclearance: immune mechanisms, clinical impact, importance for drug development. J Hepatol 2020; 73:409–422. doi: 10.1016/j.jhep.2020.04.013. [DOI] [PubMed] [Google Scholar]
- 16.Chu CM, Lin DY, Liaw YF. Does increased body mass index with hepatic steatosis contribute to seroclearance of hepatitis B virus (HBV) surface antigen in chronic HBV infection? Int J Obes (Lond) 2007; 31:871–875. doi: 10.1038/sj.ijo.0803479. [DOI] [PubMed] [Google Scholar]
- 17.Chu CM, Lin DY, Liaw YF. Clinical and virological characteristics post HBsAg seroclearance in hepatitis B virus carriers with hepatic steatosis versus those without. Dig Dis Sci 2013; 58:275–281. doi: 10.1007/s10620-012-2343-9. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Yang HI, Yeh ML, Le MH, Le AK, Yeo YH, et al. Association between fatty liver and cirrhosis, hepatocellular carcinoma, and hepatitis B surface antigen seroclearance in chronic hepatitis B. J Infect Dis 2021; 224:294–302. doi: 10.1093/infdis/jiaa739. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Le AK, Chaung KT, Henry L, Hoang JK, Cheung R, et al. Fatty liver is not independently associated with the rates of complete response to oral antiviral therapy in chronic hepatitis B patients. Liver Int 2020; 40:1052–1061. doi: 10.1111/liv.14415. [DOI] [PubMed] [Google Scholar]
- 20.Tai DI, Lin SM, Sheen IS, Chu CM, Lin DY, Liaw YF. Long-term outcome of hepatitis B e antigen-negative hepatitis B surface antigen carriers in relation to changes of alanine aminotransferase levels over time. Hepatology 2009; 49:1859–1867. doi: 10.1002/hep.22878. [DOI] [PubMed] [Google Scholar]
- 21.Tai DI, Tsay PK, Chen WT, Chu CM, Liaw YF. Relative roles of HBsAg seroclearance and mortality in the decline of HBsAg prevalence with increasing age. Am J Gastroenterol 2010; 105:1102–1109. doi: 10.1038/ajg.2009.669. [DOI] [PubMed] [Google Scholar]
- 22.Chiang CH, Yang HI, Jen CL, Lu SN, Wang LY, You SL, et al. Association between obesity, hypertriglyceridemia and low hepatitis B viral load. Int J Obes (Lond) 2013; 37:410–415. doi: 10.1038/ijo.2012.63. [DOI] [PubMed] [Google Scholar]
- 23.Hui RWH, Seto WK, Cheung KS, Mak LY, Liu KSH, Fung J, et al. Inverse relationship between hepatic steatosis and hepatitis B viremia: results of a large case-control study. J Viral Hepat 2018; 25:97–104. doi: 10.1111/jvh.12766. [DOI] [PubMed] [Google Scholar]
- 24.Wang MM, Wang GS, Shen F, Chen GY, Pan Q, Fan JG. Hepatic steatosis is highly prevalent in hepatitis B patients and negatively associated with virological factors. Dig Dis Sci 2014; 59:2571–2579. doi: 10.1007/s10620-014-3180-9. [DOI] [PubMed] [Google Scholar]
- 25.Chang JW, Lee JS, Lee HW, Kim BK, Park JY, Kim DY, et al. No influence of hepatic steatosis on the 3-year outcomes of patients with quiescent chronic hepatitis B. J Viral Hepat 2021; 28:1545–1553. doi: 10.1111/jvh.13594. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z, Pan Q, Duan XY, Liu Q, Mo GY, Rao GR, et al. Fatty liver reduces hepatitis B virus replication in a genotype B hepatitis B virus transgenic mice model. J Gastroenterol Hepatol 2012; 27:1858–1864. doi: 10.1111/j.1440-1746.2012.07268.x. [DOI] [PubMed] [Google Scholar]
- 27.Hu D, Wang H, Wang H, Wang Y, Wan X, Yan W, et al. Non-alcoholic hepatic steatosis attenuates hepatitis B virus replication in an HBV-immunocompetent mouse model. Hepatol Int 2018; 12:438–446. doi: 10.1007/s12072-018-9877-7. [DOI] [PubMed] [Google Scholar]
- 28.Zhang RN, Pan Q, Zhang Z, Cao HX, Shen F, Fan JG. Saturated fatty acid inhibits viral replication in chronic hepatitis B virus infection with nonalcoholic fatty liver disease by toll-like receptor 4-mediated innate immune response. Hepat Mon 2015; 15:e27909.doi: 10.5812/hepatmon.15(5)2015.27909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu S, Peng N, Xie J, Hao Q, Zhang M, Zhang Y, et al. Human hepatitis B virus surface and e antigens inhibit major vault protein signaling in interferon induction pathways. J Hepatol 2015; 62:1015–1023. doi: 10.1016/j.jhep.2014.11.035. [DOI] [PubMed] [Google Scholar]
- 30.Wu J, Meng Z, Jiang M, Pei R, Trippler M, Broering R, et al. Hepatitis B virus suppresses toll-like receptor-mediated innate immune responses in murine parenchymal and nonparenchymal liver cells. Hepatology 2009; 49:1132–1140. doi: 10.1002/hep.22751. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Yin S, Chen Y, Zhang Q, Huang R, Jia B, et al. Hepatitis B virus-induced hyperactivation of B cells in chronic hepatitis B patients via TLR4. J Cell Mol Med 2020; 24:6096–6106. doi: 10.1111/jcmm.15202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shlomai A, Paran N, Shaul Y. PGC-1alpha controls hepatitis B virus through nutritional signals. Proc Natl Acad Sci U S A 2006; 103:16003–16008. doi: 10.1073/pnas.0607837103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piccinin E, Villani G, Moschetta A. Metabolic aspects in NAFLD, NASH and hepatocellular carcinoma: the role of PGC1 coactivators. Nat Rev Gastroenterol Hepatol 2019; 16:160–174. doi: 10.1038/s41575-018-0089-3. [DOI] [PubMed] [Google Scholar]
- 34.Mansouri A, Gattolliat CH, Asselah T. Mitochondrial dysfunction and signaling in chronic liver diseases. Gastroenterology 2018; 155:629–647. doi: 10.1053/j.gastro.2018.06.083. [DOI] [PubMed] [Google Scholar]
- 35.Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology 2003; 125:437–443. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 36.Liu W, Lin YT, Yan XL, Ding YL, Wu YL, Chen WN, et al. Hepatitis B virus core protein inhibits Fas-mediated apoptosis of hepatoma cells via regulation of mFas/FasL and sFas expression. FASEB J 2015; 29:1113–1123. doi: 10.1096/fj.14-263822. [DOI] [PubMed] [Google Scholar]
- 37.Joo EJ, Chang Y, Yeom JS, Ryu S. Hepatitis B virus infection and decreased risk of nonalcoholic fatty liver disease: a cohort study. Hepatology 2017; 65:828–835. doi: 10.1002/hep.28917. [DOI] [PubMed] [Google Scholar]
- 38.Wong VW, Wong GL, Chu WC, Chim AM, Ong A, Yeung DK, et al. Hepatitis B virus infection and fatty liver in the general population. J Hepatol 2012; 56:533–540. doi: 10.1016/j.jhep.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 39.Zhong GC, Wu YL, Hao FB, Rao XW, Yuan XW, Zhao Y, et al. Current but not past hepatitis B virus infection is associated with a decreased risk of nonalcoholic fatty liver disease in the Chinese population: a case-control study with propensity score analysis. J Viral Hepat 2018; 25:842–852. doi: 10.1111/jvh.12878. [DOI] [PubMed] [Google Scholar]
- 40.Huang J, Jing M, Wang C, Wang M, You S, Lin S, et al. The impact of hepatitis B virus infection status on the prevalence of nonalcoholic fatty liver disease: a population-based study. J Med Virol 2020; 92:1191–1197. doi: 10.1002/jmv.25621. [DOI] [PubMed] [Google Scholar]
- 41.Wang B, Li W, Fang H, Zhou H. Hepatitis B virus infection is not associated with fatty liver disease: evidence from a cohort study and functional analysis. Mol Med Rep 2019; 19:320–326. doi: 10.3892/mmr.2018.9619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin S, Wang M, Liu Y, Huang J, Wu Y, Zhu Y, et al. Concurrence of HBV infection and non-alcoholic fatty liver disease is associated with higher prevalence of chronic kidney disease. Clin Res Hepatol Gastroenterol 2021; 45:101483.doi: 10.1016/j.clinre.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 43.Lv DD, Wang YJ, Wang ML, Chen EQ, Tao YC, Zhang DM, et al. Effect of silibinin capsules combined with lifestyle modification on hepatic steatosis in patients with chronic hepatitis B. Sci Rep 2021; 11:655.doi: 10.1038/s41598-020-80709-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng YL, Wang YJ, Kao WY, Chen PH, Huo TI, Huang YH, et al. Inverse association between hepatitis B virus infection and fatty liver disease: a large-scale study in populations seeking for check-up. PLoS One 2013; 8:e72049.doi: 10.1371/journal.pone.0072049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu L, Jiang J, Zhai X, Baecker A, Peng H, Qian J, et al. Hepatitis B virus infection and risk of non-alcoholic fatty liver disease: a population-based cohort study. Liver Int 2019; 39:70–80. doi: 10.1111/liv.13933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Viganò M, Valenti L, Lampertico P, Facchetti F, Motta BM, D’Ambrosio R, et al. Patatin-like phospholipase domain-containing 3 I148M affects liver steatosis in patients with chronic hepatitis B. Hepatology 2013; 58:1245–1252. doi: 10.1002/hep.26445. [DOI] [PubMed] [Google Scholar]
- 47.Yun JW, Cho YK, Park JH, Kim HJ, Park DI, Sohn CI, et al. Hepatic steatosis and fibrosis in young men with treatment-naive chronic hepatitis B. Liver Int 2009; 29:878–883. doi: 10.1111/j.1478-3231.2009.01976.x. [DOI] [PubMed] [Google Scholar]
- 48.Minakari M, Molaei M, Shalmani HM, Mohammad Alizadeh AH, Jazi AH, Naderi N, et al. Liver steatosis in patients with chronic hepatitis B infection: host and viral risk factors. Eur J Gastroenterol Hepatol 2009; 21:512–516. doi: 10.1097/MEG.0b013e328326792e. [DOI] [PubMed] [Google Scholar]
- 49.Pokorska-Spiewak M, Kowalik-Mikolajewska B, Aniszewska M, Pluta M, Walewska-Zielecka B, Marczynska M. Liver steatosis in children with chronic hepatitis B and C: prevalence, predictors, and impact on disease progression. Medicine (Baltimore) 2017; 96:e5832.doi: 10.1097/MD.0000000000005832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Enomoto H, Aizawa N, Nishikawa H, Ikeda N, Sakai Y, Takata R, et al. Relationship between hepatic steatosis and the elevation of aminotransferases in HBV-infected patients with HBe-antigen negativity and a low viral load. Medicine (Baltimore) 2016; 95:e3565.doi: 10.1097/MD.0000000000003565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu PT, Hwang AC, Chen JD. Combined effects of hepatitis B virus infection and elevated alanine aminotransferase levels on dyslipidemia. Metabolism 2013; 62:220–225. doi: 10.1016/j.metabol.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 52.Huang CY, Lu CW, Liu YL, Chiang CH, Lee LT, Huang KC. Relationship between chronic hepatitis B and metabolic syndrome: a structural equation modeling approach. Obesity (Silver Spring) 2016; 24:483–489. doi: 10.1002/oby.21333. [DOI] [PubMed] [Google Scholar]
- 53.Chen JY, Wang JH, Lin CY, Chen PF, Tseng PL, Chen CH, et al. Lower prevalence of hypercholesterolemia and hyperglyceridemia found in subjects with seropositivity for both hepatitis B and C strains independently. J Gastroenterol Hepatol 2010; 25:1763–1768. doi: 10.1111/j.1440-1746.2010.06300.x. [DOI] [PubMed] [Google Scholar]
- 54.Hsu CS, Liu CH, Wang CC, Tseng TC, Liu CJ, Chen CL, et al. Impact of hepatitis B virus infection on metabolic profiles and modifying factors. J Viral Hepat 2012; 19:e48–e57. doi: 10.1111/j.1365-2893.2011.01535.x. [DOI] [PubMed] [Google Scholar]
- 55.Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology 2004; 40:46–54. doi: 10.1002/hep.20280. [DOI] [PubMed] [Google Scholar]
- 56.Buechler C, Wanninger J, Neumeier M. Adiponectin, a key adipokine in obesity related liver diseases. World J Gastroenterol 2011; 17:2801–2811. doi: 10.3748/wjg.v17.i23.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoon S, Jung J, Kim T, Park S, Chwae YJ, Shin HJ, et al. Adiponectin, a downstream target gene of peroxisome proliferator-activated receptor gamma, controls hepatitis B virus replication. Virology 2011; 409:290–298. doi: 10.1016/j.virol.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 58.Hajjou M, Norel R, Carver R, Marion P, Cullen J, Rogler LE, et al. cDNA microarray analysis of HBV transgenic mouse liver identifies genes in lipid biosynthetic and growth control pathways affected by HBV. J Med Virol 2005; 77:57–65. doi: 10.1002/jmv.20427. [DOI] [PubMed] [Google Scholar]
- 59.Yilmaz B, Koklu S, Buyukbayram H, Yalcin K, Korkmaz U, Posul E, et al. Chronic hepatitis B associated with hepatic steatosis, insulin resistance, necroinflammation and fibrosis. Afr Health Sci 2015; 15:714–718. doi: 10.4314/ahs.v15i3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kang SK, Chung TW, Lee JY, Lee YC, Morton RE, Kim CH. The hepatitis B virus X protein inhibits secretion of apolipoprotein B by enhancing the expression of N-acetylglucosaminyltransferase III. J Biol Chem 2004; 279:28106–28112. doi: 10.1074/jbc.M403176200. [DOI] [PubMed] [Google Scholar]
- 61.Kim KH, Shin HJ, Kim K, Choi HM, Rhee SH, Moon HB, et al. Hepatitis B virus X protein induces hepatic steatosis via transcriptional activation of SREBP1 and PPARgamma. Gastroenterology 2007; 132:1955–1967. doi: 10.1053/j.gastro.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 62.Wu YL, Peng XE, Zhu YB, Yan XL, Chen WN, Lin X. Hepatitis B virus X protein induces hepatic steatosis by enhancing the expression of liver fatty acid binding protein. J Virol 2016; 90:1729–1740. doi: 10.1128/JVI.02604-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krawczyk M, Rau M, Schattenberg JM, Bantel H, Pathil A, Demir M, et al. Combined effects of the PNPLA3 rs738409, TM6SF2 rs58542926, and MBOAT7 rs641738 variants on NAFLD severity: a multicenter biopsy-based study. J Lipid Res 2017; 58:247–255. doi: 10.1194/jlr.P067454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eslam M, Valenti L, Romeo S. Genetics and epigenetics of NAFLD and NASH: clinical impact. J Hepatol 2018; 68:268–279. doi: 10.1016/j.jhep.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 65.Pan Q, Chen MM, Zhang RN, Wang YQ, Zheng RD, Mi YQ, et al. PNPLA3 rs1010023 predisposes chronic hepatitis B to hepatic steatosis but improves insulin resistance and glucose metabolism. J Diabetes Res 2017; 2017:4740124.doi: 10.1155/2017/4740124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eslam M, Mangia A, Berg T, Chan HL, Irving WL, Dore GJ, et al. Diverse impacts of the rs58542926 E167K variant in TM6SF2 on viral and metabolic liver disease phenotypes. Hepatology 2016; 64:34–46. doi: 10.1002/hep.28475. [DOI] [PubMed] [Google Scholar]
- 67.Kleiner DE, Brunt EM, Wilson LA, Behling C, Guy C, Contos M, et al. Association of histologic disease activity with progression of nonalcoholic fatty liver disease. JAMA Netw Open 2019; 2:e1912565.doi: 10.1001/jamanetworkopen.2019.12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Caines A, Selim R, Salgia R. The changing global epidemiology of hepatocellular carcinoma. Clin Liver Dis 2020; 24:535–547. doi: 10.1016/j.cld.2020.06.001. [DOI] [PubMed] [Google Scholar]
- 69.Cheng JY, Wong VW, Tse YK, Chim AM, Chan HL, Wong GL. Metabolic syndrome increases cardiovascular events but not hepatic events and death in patients with chronic hepatitis B. Hepatology 2016; 64:1507–1517. doi: 10.1002/hep.28778. [DOI] [PubMed] [Google Scholar]
- 70.Chen Y, Fan C, Chen Y, Liu H, Wang S, Dong P, et al. Effect of hepatic steatosis on the progression of chronic hepatitis B: a prospective cohort and in vitro study. Oncotarget 2017; 8:58601–58610. doi: 10.18632/oncotarget.17380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pais R, Rusu E, Zilisteanu D, Circiumaru A, Micu L, Voiculescu M, et al. Prevalence of steatosis and insulin resistance in patients with chronic hepatitis B compared with chronic hepatitis C and non-alcoholic fatty liver disease. Eur J Intern Med 2015; 26:30–36. doi: 10.1016/j.ejim.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 72.Karacaer Z, Okur G, Cermik H, Altun D. Is there an influence of hepatic steatosis on fibrosis and necroinflammation in young patients with chronic viral hepatitis B? Postgrad Med 2016; 128:697–700. doi: 10.1080/00325481.2016.1221733. [DOI] [PubMed] [Google Scholar]
- 73.Lim CT, Goh GBB, Li H, Lim TK, Leow WQ, Wan WK, et al. Presence of hepatic steatosis does not increase the risk of hepatocellular carcinoma in patients with chronic hepatitis B over long follow-up. Microbiol Insights 2020; 13:1178636120918878.doi: 10.1177/1178636120918878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khalili M, Kleiner DE, King WC, Sterling RK, Ghany MG, Chung RT, et al. Hepatic steatosis and steatohepatitis in a large north american cohort of adults with chronic hepatitis B. Am J Gastroenterol 2021; 116:1686–1697. doi: 10.14309/ajg.0000000000001257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seto WK, Hui RWH, Mak LY, Fung J, Cheung KS, Liu KSH, et al. Association between hepatic steatosis, measured by controlled attenuation parameter, and fibrosis burden in chronic hepatitis B. Clin Gastroenterol Hepatol 2018; 16:575–583.e2. doi: 10.1016/j.cgh.2017.09.044. [DOI] [PubMed] [Google Scholar]
- 76.Charatcharoenwitthaya P, Pongpaibul A, Kaosombatwattana U, Bhanthumkomol P, Bandidniyamanon W, Pausawasdi N, et al. The prevalence of steatohepatitis in chronic hepatitis B patients and its impact on disease severity and treatment response. Liver Int 2017; 37:542–551. doi: 10.1111/liv.13271. [DOI] [PubMed] [Google Scholar]
- 77.Mak LY, Hui RW, Fung J, Liu F, Wong DK, Cheung KS, et al. Diverse effects of hepatic steatosis on fibrosis progression and functional cure in virologically quiescent chronic hepatitis B. J Hepatol 2020; 73:800–806. doi: 10.1016/j.jhep.2020.05.040. [DOI] [PubMed] [Google Scholar]
- 78.Wong SW, Chan WK, Mohamed R. Fatty liver is associated with advanced fibrosis but does not predict adverse outcomes in patients with chronic hepatitis B. J Viral Hepat 2020; 27:1297–1305. doi: 10.1111/jvh.13361. [DOI] [PubMed] [Google Scholar]
- 79.van Kleef LA, Choi HSJ, Brouwer WP, Hansen BE, Patel K, de Man RA, et al. Metabolic dysfunction-associated fatty liver disease increases risk of adverse outcomes in patients with chronic hepatitis B. JHEP Rep 2021; 3:100350.doi: 10.1016/j.jhepr.2021.100350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim MN, Han K, Yoo J, Hwang SG, Ahn SH. Increased risk of hepatocellular carcinoma and mortality in chronic viral hepatitis with concurrent fatty liver. Aliment Pharmacol Ther 2022; 55:97–107. doi: 10.1111/apt.16706. [DOI] [PubMed] [Google Scholar]
- 81.Peleg N, Issachar A, Sneh Arbib O, Cohen-Naftaly M, Braun M, Leshno M, et al. Liver steatosis is a strong predictor of mortality and cancer in chronic hepatitis B regardless of viral load. JHEP Rep 2019; 1:9–16. doi: 10.1016/j.jhepr.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Massoud O, Charlton M. Nonalcoholic fatty liver disease/nonalcoholic steatohepatitis and hepatocellular carcinoma. Clin Liver Dis 2018; 22:201–211. doi: 10.1016/j.cld.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 83.Streba LA, Vere CC, Rogoveanu I, Streba CT. Nonalcoholic fatty liver disease, metabolic risk factors, and hepatocellular carcinoma: an open question. World J Gastroenterol 2015; 21:4103–4110. doi: 10.3748/wjg.v21.i14.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chung YL, Wu ML. The role of promyelocytic leukemia protein in steatosis-associated hepatic tumors related to chronic hepatitis B virus infection. Transl Oncol 2018; 11:743–754. doi: 10.1016/j.tranon.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Eden A, Gaudet F, Waghmare A, Jaenisch R. Chromosomal instability and tumors promoted by DNA hypomethylation. Science 2003; 300:455.doi: 10.1126/science.1083557. [DOI] [PubMed] [Google Scholar]
- 86.Zeybel M, Vatansever S, Hardy T, Sari AA, Cakalagaoglu F, Avci A, et al. DNA methylation profiling identifies novel markers of progression in hepatitis B-related chronic liver disease. Clin Epigenetics 2016; 8:48.doi: 10.1186/s13148-016-0218-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li F, Ou Q, Lai Z, Pu L, Chen X, Wang L, et al. The co-occurrence of chronic hepatitis B and fibrosis is associated with a decrease in hepatic global DNA methylation levels in patients with non-alcoholic fatty liver disease. Front Genet 2021; 12:671552.doi: 10.3389/fgene.2021.671552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim DS, Jeon MY, Lee HW, Kim BK, Park JY, Kim DY, et al. Influence of hepatic steatosis on the outcomes of patients with chronic hepatitis B treated with entecavir and tenofovir. Clin Mol Hepatol 2019; 25:283–293. doi: 10.3350/cmh.2018.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhu LY, Wang YG, Wei LQ, Zhou J, Dai WJ, Zhang XY. The effects of the insulin resistance index on the virologic response to entecavir in patients with HBeAg-positive chronic hepatitis B and nonalcoholic fatty liver disease. Drug Des Devel Ther 2016; 10:2739–2744. doi: 10.2147/DDDT.S114761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dogan Z, Filik L, Ergul B, Sarikaya M. Comparison of first-year results of tenofovir and entecavir treatments of nucleos(t)ide-naive chronic hepatitis B patients with hepatosteatosis. Saudi J Gastroenterol 2015; 21:396–399. doi: 10.4103/1319-3767.164186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cindoruk M, Karakan T, Unal S. Hepatic steatosis has no impact on the outcome of treatment in patients with chronic hepatitis B infection. J Clin Gastroenterol 2007; 41:513–517. doi: 10.1097/01.mcg.0000225586.78330.60. [DOI] [PubMed] [Google Scholar]
- 92.Chen YC, Jeng WJ, Hsu CW, Lin CY. Impact of hepatic steatosis on treatment response in nuclesos(t)ide analogue-treated HBeAg-positive chronic hepatitis B: a retrospective study. BMC Gastroenterol 2020; 20:146.doi: 10.1186/s12876-020-01289-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ceylan B, Arslan F, Batirel A, Fincanci M, Yardimci C, Fersan E, et al. Impact of fatty liver on hepatitis B virus replication and virologic response to tenofovir and entecavir. Turk J Gastroenterol 2016; 27:42–46. doi: 10.5152/tjg.2015.150348. [DOI] [PubMed] [Google Scholar]
- 94.Liang H, Liu Y, Jiang X, Zheng X, Tang J, Yang J, et al. Impact of hepatic steatosis on the antiviral effects of PEG-IFNalpha-2a in patients with chronic hepatitis B and the associated mechanism. Gastroenterol Res Pract 2020; 2020:1794769.doi: 10.1155/2020/1794769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jin X, Chen YP, Yang YD, Li YM, Zheng L, Xu CQ. Association between hepatic steatosis and entecavir treatment failure in Chinese patients with chronic hepatitis B. PLoS One 2012; 7:e34198.doi: 10.1371/journal.pone.0034198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhu Y, Yang Q, Lv F, Yu Y. The effect of hepatosteatosis on response to antiviral treatment in patients with chronic hepatitis B: a meta-analysis. Gastroenterol Res Pract 2017; 2017:1096406.doi: 10.1155/2017/1096406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yao J, Zhou L, Hua X, Kong M, Chen Y, Duan Z. Effects of nucleos(t)ide analogs on body composition in HBV-infected men: an age- and BMI-matched, cross-sectional study. Nutrition 2016; 32:1206–1210. doi: 10.1016/j.nut.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 98.Madeddu G, Soddu A, Mannu F, Muredda AA, Garrucciu G, Bandiera F, et al. Body fat changes and mitochondrial alterations during HBV treatment: a warning for long term administration. J Infect 2012; 65:467–470. doi: 10.1016/j.jinf.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 99.Suzuki K, Suda G, Yamamoto Y, Furuya K, Baba M, Nakamura A, et al. Tenofovir-disoproxil-fumarate modulates lipid metabolism via hepatic CD36/PPAR-alpha activation in hepatitis B virus infection. J Gastroenterol 2021; 56:168–180. doi: 10.1007/s00535-020-01750-3. [DOI] [PubMed] [Google Scholar]
- 100.Sripongpun P, Kim WR, Mannalithara A, Kwong A, Daugherty T, Goel A, et al. Tenofovir alafenamide attenuates effects of diabetes and body mass on serum alanine aminotransferase activities in patients with chronic hepatitis B. Clin Gastroenterol Hepatol 2022; 20:230–232. doi: 10.1016/j.cgh.2020.11.047. [DOI] [PubMed] [Google Scholar]
- 101.Kim GA, Shim JJ, Lee JS, Kim BH, Kim JW, Oh CH, et al. Effect of statin use on liver cancer mortality considering hypercholesterolemia and obesity in patients with non-cirrhotic chronic hepatitis B. Yonsei Med J 2019; 60:1203–1208. doi: 10.3349/ymj.2019.60.12.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gripon P, Diot C, Theze N, Fourel I, Loreal O, Brechot C, et al. Hepatitis B virus infection of adult human hepatocytes cultured in the presence of dimethyl sulfoxide. J Virol 1988; 62:4136–4143. doi: 10.1128/JVI.62.11.4136-4143.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Verrier ER, Colpitts CC, Schuster C, Zeisel MB, Baumert TF. Cell culture models for the investigation of hepatitis B and D virus infection. Viruses 2016; 8:261.doi: 10.3390/v8090261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gripon P, Rumin S, Urban S, Le Seyec J, Glaise D, Cannie I, et al. Infection of a human hepatoma cell line by hepatitis B virus. Proc Natl Acad Sci U S A 2002; 99:15655–15660. doi: 10.1073/pnas.232137699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sells MA, Chen ML, Acs G. Production of hepatitis B virus particles in Hep G2 cells transfected with cloned hepatitis B virus DNA. Proc Natl Acad Sci U S A 1987; 84:1005–1009. doi: 10.1073/pnas.84.4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chang CM, Jeng KS, Hu CP, Lo SJ, Su TS, Ting LP, et al. Production of hepatitis B virus in vitro by transient expression of cloned HBV DNA in a hepatoma cell line. EMBO J 1987; 6:675–680. doi: 10.1002/j.1460-2075.1987.tb04807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ni Y, Lempp FA, Mehrle S, Nkongolo S, Kaufman C, Falth M, et al. Hepatitis B and D viruses exploit sodium taurocholate co-transporting polypeptide for species-specific entry into hepatocytes. Gastroenterology 2014; 146:1070–1083. doi: 10.1053/j.gastro.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 108.Wieland SF. The chimpanzee model for hepatitis B virus infection. Cold Spring Harb Perspect Med 2015; 5:a021469.doi: 10.1101/cshperspect.a021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Klumpp K, Shimada T, Allweiss L, Volz T, Lutgehetmann M, Hartman G, et al. Efficacy of NVR 3-778, alone and in combination with pegylated interferon, vs entecavir in uPA/SCID mice with humanized livers and HBV infection. Gastroenterology 2018; 154:652–662.e8. doi: 10.1053/j.gastro.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 110.Dandri M, Burda MR, Torok E, Pollok JM, Iwanska A, Sommer G, et al. Repopulation of mouse liver with human hepatocytes and in vivo infection with hepatitis B virus. Hepatology 2001; 33:981–988. doi: 10.1053/jhep.2001.23314. [DOI] [PubMed] [Google Scholar]
- 111.Lai F, Wee CYY, Chen Q. Establishment of humanized mice for the study of HBV. Front Immunol 2021; 12:638447.doi: 10.3389/fimmu.2021.638447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Guidotti LG, Matzke B, Schaller H, Chisari FV. High-level hepatitis B virus replication in transgenic mice. J Virol 1995; 69:6158–6169. doi: 10.1128/JVI.69.10.6158-6169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Peng XH, Ren XN, Chen LX, Shi BS, Xu CH, Fang Z, et al. High persistence rate of hepatitis B virus in a hydrodynamic injection-based transfection model in C3H/HeN mice. World J Gastroenterol 2015; 21:3527–3536. doi: 10.3748/wjg.v21.i12.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang PL, Althage A, Chung J, Chisari FV. Hydrodynamic injection of viral DNA: a mouse model of acute hepatitis B virus infection. Proc Natl Acad Sci U S A 2002; 99:13825–13830. doi: 10.1073/pnas.202398599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li F, Wang Z, Hu F, Su L. Cell culture models and animal models for HBV study. Adv Exp Med Biol 2020; 1179:109–135. doi: 10.1007/978-981-13-9151-4_5. [DOI] [PubMed] [Google Scholar]
- 116.Im YR, Hunter H, de Gracia Hahn D, Duret A, Cheah Q, Dong J, et al. A systematic review of animal models of NAFLD finds high-fat, high-fructose diets most closely resemble human NAFLD. Hepatology 2021; 74:1884–1901. doi: 10.1002/hep.31897. [DOI] [PubMed] [Google Scholar]
- 117.Asgharpour A, Cazanave SC, Pacana T, Seneshaw M, Vincent R, Banini BA, et al. A diet-induced animal model of non-alcoholic fatty liver disease and hepatocellular cancer. J Hepatol 2016; 65:579–588. doi: 10.1016/j.jhep.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Weltman MD, Farrell GC, Liddle C. Increased hepatocyte CYP2E1 expression in a rat nutritional model of hepatic steatosis with inflammation. Gastroenterology 1996; 111:1645–1653. doi: 10.1016/s0016-5085(96)70028-8. [DOI] [PubMed] [Google Scholar]