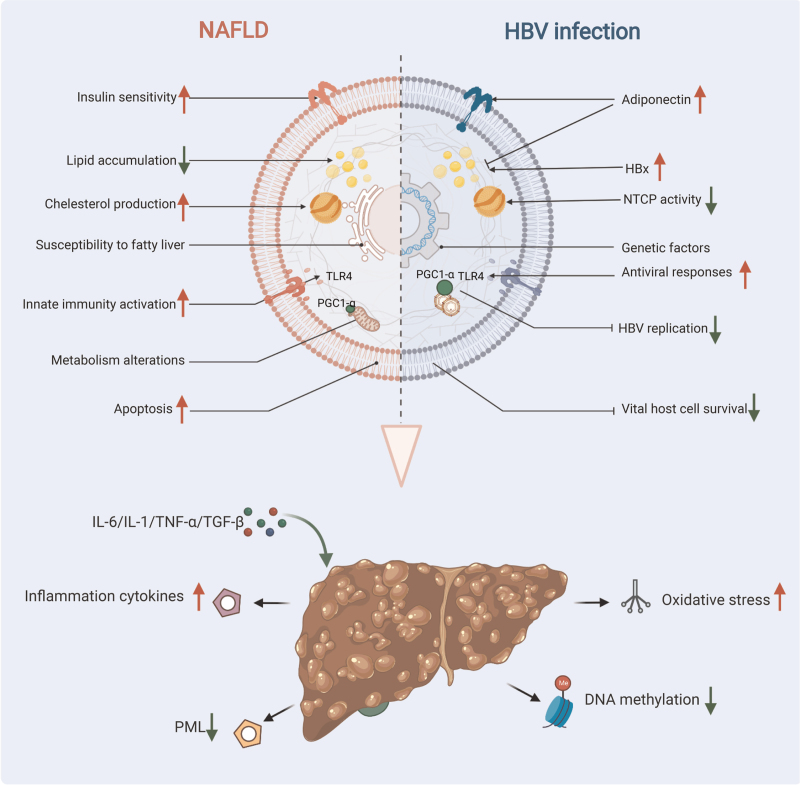
Figure 1.
Potential mechanisms of interaction between NAFLD and CHB and clinical outcomes. Metabolic alterations in NAFLD patients may enhance antiviral responses through activation of innate immunity, such as by activating the TLR4 signaling pathway or suppressing the expression of PGC-1α. NAFLD could also accelerate HBsAg and HBV-DNA clearance by increasing apoptosis in HBV-infected cells. Hepatic steatosis in CHB patients is associated with host metabolic factors, a reduced risk of hyperlipidemia has been observed in patients with CHB. In addition, the higher serum adiponectin level could account for the lower prevalence of steatosis in HBV-infected subjects. Conversely, the overexpression of HBx and the genetic susceptibility to fatty liver in CHB patients induce hepatic lipid accumulation. There are many potential mechanisms for clinical outcomes in NAFLD combined with CHB. Inflammatory cytokines (eg, IL-1, IL-6, TNF-α, TGFβ) released from damaged hepatocytes in NASH can lead to activation and proliferation of hepatic stellate cells. NAFLD-related fat deposition and oxidative stress may create a pro-fibrotic and pro-carcinogenic milieu within the liver. In addition, the PML deficiency-mediated abnormal lipid metabolism induced by HBsAg and the lower levels of global DNA methylation in patients with concurrent NAFLD and CHB could both accelerate the development of cirrhosis and HCC. CHB: Chronic hepatitis B; HBsAg: Hepatitis B surface antigen; HBV: hepatitis B virus; HBx: Hepatitis B protein X; NAFLD: Non-alcoholic fatty liver disease; NASH: Non-alcoholic steatohepatitis; PGC-1α: Peroxisome proliferator-activated receptor-γ coactivator-1α; PML: Promyelocyticleukemia protein; TLR4: Toll-like receptor 4; NTCP: Sodium taurocholate cotransporting polypeptide; IL-1: Interleukin-1; IL-6: Interleukin-6; TNF-α: Tumor necrosis factor-α; TGFβ: Transforming growth factor β.