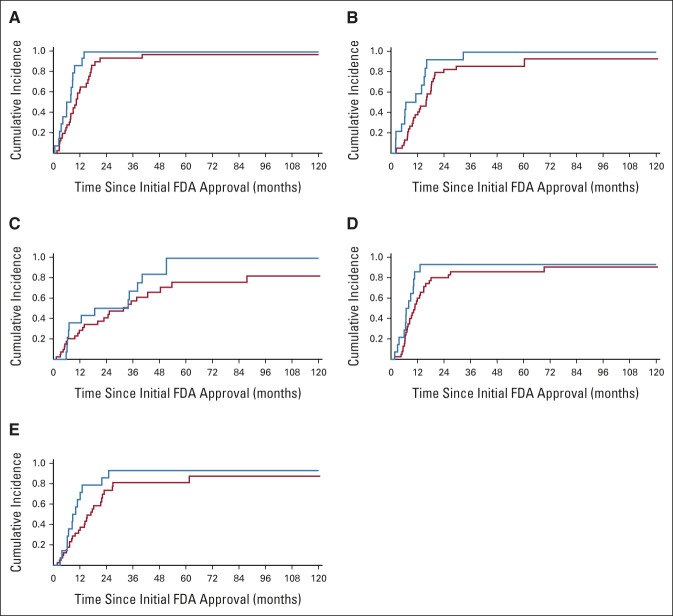
FIG 2.
Association between time to subsequent regulatory approval and clinical benefit according to ESMO-MCBS: (A) EMA, (B) Swissmedic, (C) PMDA, (D) Health Canada, and (E) TGA. High clinical benefit is shown in blue; low clinical benefit is shown in red. EMA, European Medicines Agency; ESMO-MCBS, European Society for Medical Oncology Magnitude of Clinical Benefit Scale; FDA, US Food and Drug Administration; PMDA, Pharmaceuticals and Medical Devices Agency; TGA, Therapeutic Goods Administration.