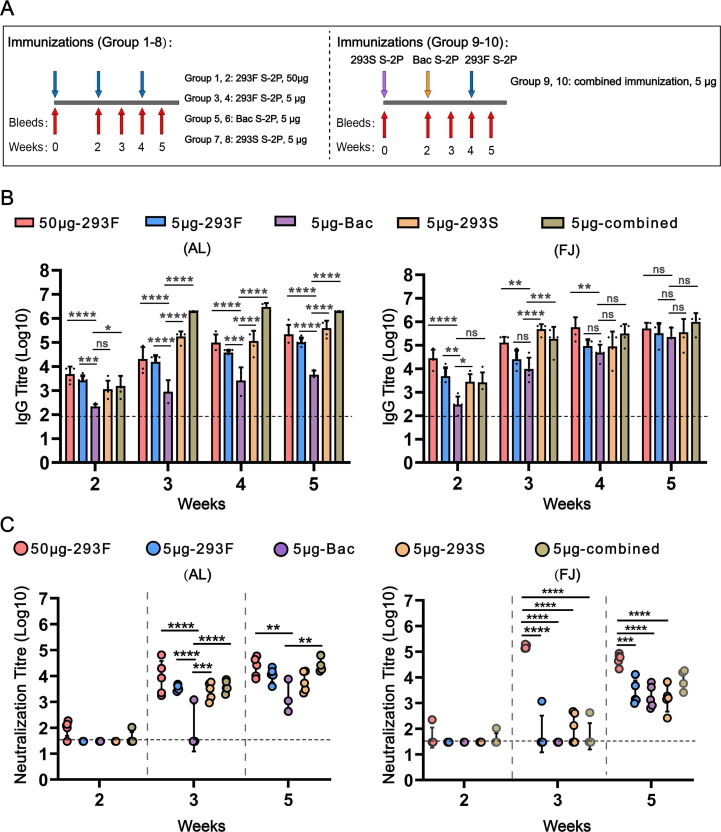
Fig. 4.
Immunogenicity of SARS-CoV-2 spike (S) proteins. (A) Mice immunization schedules. Group 1, 3, 5, 7, 9 received immunizations formulated with aluminium adjuvant. Group 2, 4, 6, 8, 10 received immunizations formulated with Freund’s adjuvant. (B) Antigen-specific IgG antibody titres induced by ten immunized groups. (C) Neutralizing antibody titres against SARS-CoV-2 pseudovirus (Wuhan-Hu-1 strain). The dotted line indicates the limit of detection for the assay. Statistical analysis was performed by one-way ANOVA. Significant differences were determined using the Holm-Sidak pairwise multiple comparisons in Graphpad Prism 8.0 (ns = non-significant; *P < 0.1, **P < 0.01, ***P < 0.001, ****P < 0.0001). (A) Groups statistically different from the Bac S-2P group are indicated. (B) Groups statistically different from each other are indicated. An absence of symbols indicates no statistical difference between the groups.