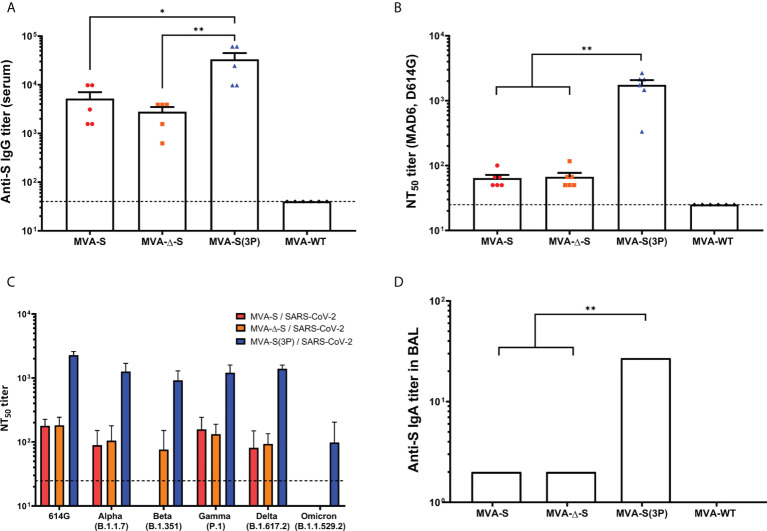
Figure 1.
SARS-CoV-2-specific humoral immune responses elicited in C57BL/6 mice immunized with one IN dose of different MVA-based vaccine candidates against COVID-19. SARS-CoV-2-specific humoral immune responses were evaluated in serum and BAL samples obtained at 14 days postimmunization from C57BL/6 mice immunized with one IN dose of MVA-S, MVA-Δ-S, or MVA-S(3P). (A) Titers of binding IgG antibodies specific for the S protein (Wuhan strain), determined by ELISA in individual mouse serum samples in duplicate. Mean values and SEM are represented. The dashed line represents the limit of detection. (B) SARS-CoV-2 neutralizing antibody titers. NT50 titers were determined in individual mouse serum samples by using a live virus microneutralization assay. Mean NT50 values and SEM are represented. The dashed line represents the limit of detection (1:25 dilution). (C) SARS-CoV-2 neutralizing antibody titers against SARS-CoV-2 VoC. NT50 titers were evaluated in pooled mouse serum samples, using VSV-based pseudoparticles expressing the SARS-CoV-2 S protein of different VoC. Mean NT50 values and 95% confidence intervals are represented. The dashed line represents the limit of detection. (D) Titers of IgA antibodies specific for the S protein (Wuhan strain), determined by ELISA in pooled BAL samples in duplicate. Mean values and SEM are represented. Student’s t-test: *p < 0.05; **p < 0.005.