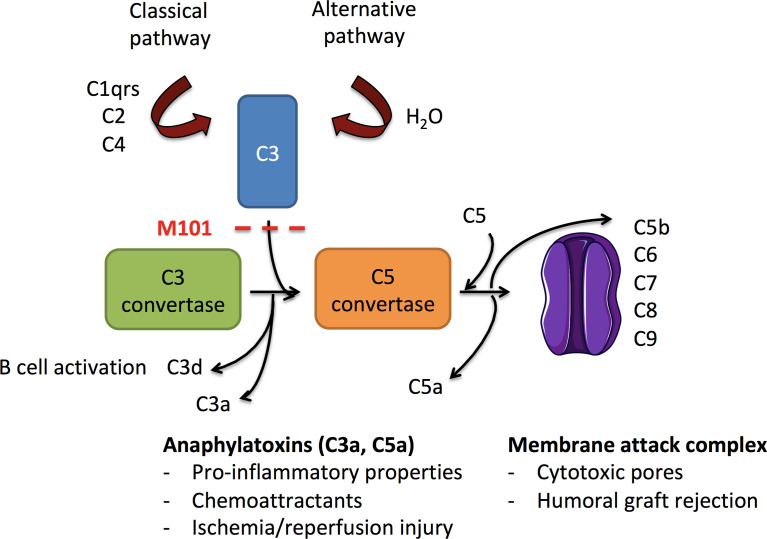
Figure 1.
Schematic overview of complement activation in ischemia/reperfusion injury and allograft rejection. Activated following immunoglobulin binding to its antigen (classical pathway) or after the continuous hydrolysis of C3 in an aqueous environment (alternative pathway), both complement pathways lead to the formation of C3 convertases (C4bC2a and C3bBb, respectively). Downstream, C3 convertases cleave C3 into C3a (anaphylatoxin) and C3b. C3b amplifies the complement activation through formation of new C3 convertases and contributes to the formation of C5 convertases (C4bC2aC3b and C3bBbC3b). C3 convertase is further inhibited by factors I and H that finally convert C3b into C3d. C5 convertases cleave C5 into the anaphylatoxin C5a and C5b to generate the membrane attack complex (MAC, C6-C9) and the release of sC5b-9. C3a and C5a possess anaphylatoxin properties, and C3d provides ligands for complement receptors (CR)2 present on B cells. As a consequence, the use of M101 in transplantation may protect from antibody binding (classical pathway), alternative pathway activation, and subsequent effector functions.