FIGURE 1.
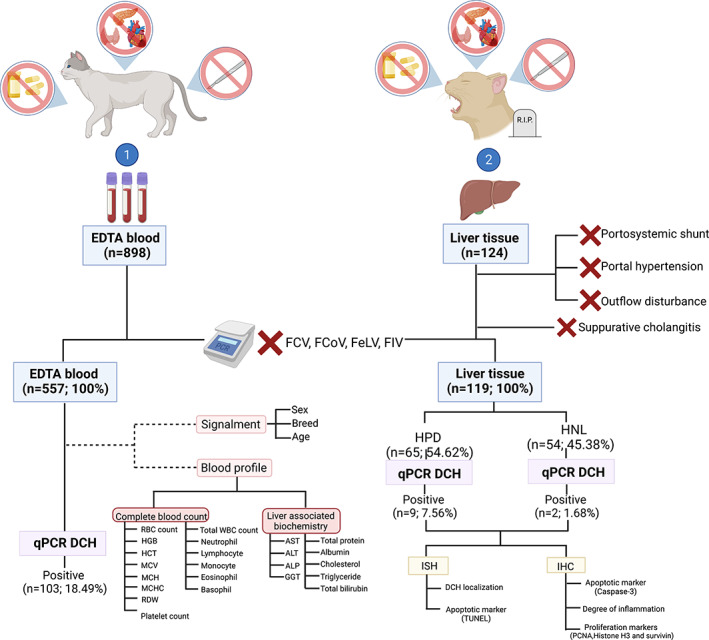
Research design and sample collection for DCH investigations. Two sample cohorts including EDTA blood and liver tissues from cats that (a) had no evidence of concurrent diseases (such as hyperthyroidism, pancreatitis, and cardiovascular disorders); (b) had not been treated with corticosteroids, antifungal drugs, or anti‐seizure medication within 3 months before sample collection; and (c) had not undergone an operation within the previous month were employed for this study. For necropsied cases, the cats that had postmortem evidence of portosystemic shunt, portal hypertension, and hepatic outflow disturbances (such as hepatic veno‐occlusive disease, obstructive bile flow diseases, etc) were discarded. The liver samples that showed histologically present suppurative cholangitis was also ruled out. The blood and liver samples in which FCV, FCoV, FeLV, or FIV was co‐detected by RT‐PCR were also excluded, and the remaining samples were further screened for DCH using qPCR. Information regarding the complete blood count (CBC) and liver biochemical profiles was collected for statistical analysis. Two sets of DCH‐positive liver samples (HPD and HNL) subsequently underwent ISH targeting the DCH partial gene to indicate the tropism of the virus; additionally, the proliferative and apoptotic activities of hepatocytes were investigated using apoptotic (caspase‐3 and terminal deoxynucleotidyl transferase dUTP nick‐end labeling [TUNEL] assays) and proliferative (PCNA, phospho‐histone H3, and survivin) panels
