Abstract
Background
This study’s primary aim was to evaluate the impact of thrombotic complications on the development of secondary infections. The secondary aim was to compare the etiology of secondary infections in patients with and without thrombotic complications.
Methods
This was a cohort study (NCT04318366) of coronavirus disease 2019 (COVID-19) patients hospitalized at IRCCS San Raffaele Hospital between February 25 and June 30, 2020. Incidence rates (IRs) were calculated by univariable Poisson regression as the number of cases per 1000 person-days of follow-up (PDFU) with 95% confidence intervals. The cumulative incidence functions of secondary infections according to thrombotic complications were compared with Gray's method accounting for competing risk of death. A multivariable Fine-Gray model was applied to assess factors associated with risk of secondary infections.
Results
Overall, 109/904 patients had 176 secondary infections (IR, 10.0; 95% CI, 8.8–11.5; per 1000-PDFU). The IRs of secondary infections among patients with or without thrombotic complications were 15.0 (95% CI, 10.7–21.0) and 9.3 (95% CI, 7.9–11.0) per 1000-PDFU, respectively (P = .017). At multivariable analysis, thrombotic complications were associated with the development of secondary infections (subdistribution hazard ratio, 1.788; 95% CI, 1.018–3.140; P = .043). The etiology of secondary infections was similar in patients with and without thrombotic complications.
Conclusions
In patients with COVID-19, thrombotic complications were associated with a high risk of secondary infections.
Keywords: thrombosis, bacteria, coronavirus, infections, pulmonary embolism
Coronavirus disease 2019 (COVID-19)–associated coagulopathy leading to venous and arterial thrombotic complications is frequent and potentially life-threatening in patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [1–3]. Indeed, COVID-19 has been associated with a dysregulated inflammatory response [4] and a hypercoagulable state [5], with studies reporting thrombotic events in up to 30% of patients [6]. Therefore, several approaches have been proposed regarding the best management of anticoagulation in patients with COVID-19, albeit without reaching a definitive conclusion [7, 8]. Another critical issue in hospitalized COVID-19 patients is represented by the high frequency of secondary infections, especially in patients with need of intensive care [9–12].
Interestingly, several studies have shown that immune activation during COVID-19 is frequently accompanied by immune exhaustion [13, 14]. Together with lymphopenia [15], this state of immune dysregulation may also lead to suppression of the immune response and, possibly, development of secondary infections [16]. Moreover, while systemic infections are a known risk factor for thrombotic complications [17], thrombotic lesions are known to be at risk of bacterial colonization and subsequent development of infection, as exemplified by infective endocarditis [18]. Given the possible shared pathophysiological pathway between coagulation disorders, derangement of the immune system, and infectious complications, we aimed at evaluating the impact of thrombotic complications on the development of secondary infections. Furthermore, we compared the etiology of secondary infections in patients with and without thrombotic complications.
METHODS
Study Population
Patients considered in this analysis are part of the COVID-19 prospective institutional cohort (COVID-BioB) at the Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) San Raffaele Hospital, a 1350-bed tertiary care hospital in Milan, Italy. We included all patients hospitalized with COVID-19 between February 25 and June 30, 2020.
COVID-19 was defined as a positive real-time reverse transcriptase polymerase chain reaction for SARS-CoV-2 from a nasopharyngeal swab or bronchoalveolar lavage associated with suggestive signs, symptoms, and/or radiological findings. Patients were managed according to internal institutional guidelines and the available evidence at the time of admission. Antithrombotic prophylaxis or treatment was not universally administered but was introduced on a case-by-case basis according to the treating physician.
Thrombotic complications (superficial, deep venous and arterial thrombosis [SVT, DVT, AT], pulmonary thrombosis/thromboembolism [PT]) were considered to precede secondary infections if diagnosed before or <48 hours after microbiological evidence of infection. All thrombotic complications had to be documented by either contrast-enhanced computed tomography (CT) scan or doppler ultrasonography (US); scans were requested at the treating physician's discretion. Heparin use included either prophylactic or full-dose anticoagulation.
Secondary infections (either bloodstream infections [BSIs] or lower respiratory tract infections [LRTIs]) were included in the analysis only if occurring at least 48 hours after hospital admission in order to differentiate them from coinfections.
BSIs were defined as a single positive blood culture for a likely pathogen or 2 or more positive blood cultures for common skin colonizers (ie, coagulase-negative staphylococci, diphtheroids, Bacillus spp., Cutibacterium spp., viridans group streptococci), without a concomitant microbiologically documented lower respiratory tract infection due to the same pathogen. Patients who had >1 positive blood culture within 7 days from the first positive blood culture were considered to have a single episode of BSI with multiple isolates.
LRTIs were defined as positive cultures of potentially pathogenic organisms from respiratory specimens obtained with invasive techniques (bronchoscopy-guided bronchoalveolar lavage [BAL] or, when not available, bronchial aspirate [BRASP]), excluding Candida spp. COVID-19-associated pulmonary aspergillosis was defined according to the ECMM/ISHAM consensus criteria [19].
Both blood and respiratory cultures were requested by the attending physicians in patients with suspected secondary infections because of the clinical and/or respiratory deterioration associated with suggestive laboratory or radiological findings. BAL and BRASP were not routinely collected for surveillance.
Patients for whom no microbiology specimens were requested were considered not to have secondary infections.
Microbiological Methods
Blood culture samples were processed using the bioMérieux Virtuo BacT/Alert system, respiratory samples were inoculated in specific growth media (according to site protocol) and identified using Matrix Assisted Laser Desorption/Ionisation Time-Of-Flight Mass Spectrometry (MALDI-TOF MS; VITEK-MS, bioMérieux). The Platelia Aspergillus immuno-enzymatic assay was used to detect galactomannan.
Statistical Analysis
Results of continuous variables were described by median (quartiles), while categorical variables were described by frequency (%).
The characteristics of patients with or without at least 1 secondary infection during hospitalization were compared with the chi-square or Fisher exact test for categorical variables and the Mann-Whitney U test for continuous variables. The distribution of microbial etiology of secondary infections among patients with or without thrombotic complications was compared with the chi-square test.
As previously described [20], quartiles of ferritin, C-reactive protein (CRP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), D-dimer, and prothrombin time were calculated in order to determine (i) a composite inflammation score (number of elevated biomarkers of inflammation, ie, ≥75th percentile threshold of ferritin and CRP); (ii) a composite cytolysis score (number of elevated biomarkers of cytolysis, ie, ≥75th percentile threshold of AST, ALT, LDH); (iii) a composite coagulation score (number of elevated biomarkers of coagulation, ie, ≥75th percentile threshold of D-dimer and prothrombin time). The 3 scores were thus defined as the number of laboratory parameters with markedly elevated values (values at or above the 75th percentile), ranging from 0 to 2 (in case of inflammation and coagulation) or 3 (in case of cytolysis score).
Incidence rates of secondary infections and thrombotic complications were calculated by univariable Poisson regression and reported as number of cases per 1000 person-days of follow-up (PDFUs).
The cumulative incidence function (CIF) of 1 or more secondary infections was calculated in the overall cohort and according to the presence of thrombotic complications with Gray's method [21]; 95% confidence intervals for survival probabilities and cumulative incidence were calculated accounting for competing risks of death.
A multivariable Fine-Gray model was applied to assess factors associated with the risk of secondary infections, including all variables deemed to be clinically significant or with a P value <.200 at univariate analysis, along with the 3 previously described scores based on laboratory parameters. Moreover, a sensitivity analysis including use of heparin before the diagnosis of secondary infections was performed.
All statistical tests were 2-sided at the 5% level and were performed using SAS 9.4 (Statistical Analyses System Inc, Cary, NC, USA).
Patient Consent
Written informed consent was obtained according to institutional review board guidelines. The study is part of the institutional clinical–biological cohort assessing patients with COVID-19 (COVID-BioB, ClinicalTrials.gov NCT04318366), which was approved by the Institutional Review Board of IRCCS San Raffaele Hospital (protocol number 34/int/2020).
RESULTS
Patients’ Characteristics
Overall, 904 patients were included in the study. Patients’ characteristics and baseline (hospital admission) laboratory parameters are described in Table 1. All-cause in-hospital mortality was 22% (199/904 patients), with a median time to death since hospital admission (interquartile range [IQR]) of 13 (7–22) days. The median duration of hospital stay (IQR) was 14 (8–25) days.
Table 1.
Patients’ Characteristics and Baseline Laboratory Parameters
| Characteristics | Category | Overall (n = 904) |
With Secondary Infections (n = 109) |
Without Secondary Infections (n = 795) |
P Valuea |
|---|---|---|---|---|---|
| Age, y | … | 64 (55–76) | 63 (56–71) | 65 (54–76) | .902 |
| Sex, female | … | 297 (32.9%) | 22 (20.2%) | 275 (34.6%) | .002 |
| Arterial hypertension | … | 400 (45.4%) | 38 (39.6%) | 362 (46.1%) | .234 |
| Coronary artery disease | … | 226 (25.7%) | 20 (20.8%) | 206 (26.2%) | .268 |
| Diabetes mellitus | … | 158 (17.9%) | 21 (21.6%) | 137 (17.5%) | .326 |
| Chronic obstructive pulmonary disease | … | 64 (7.3%) | 3 (3.1%) | 61 (7.8%) | .100 |
| Chronic kidney disease | … | 87 (9.9%) | 8 (8.2%) | 79 (10.1%) | .718 |
| Malignancy | … | 133 (15.1%) | 7 (7.3%) | 126 (16.1%) | .022 |
| Liver disease | … | 17 (2.9%) | 3 (6.5%) | 14 (2.6%) | .143 |
| No. of comorbidities | … | 1 (0–2) | 1 (0–2) | 1 (0–2) | .122 |
| Days from symptom onset to hospital admission | … | 7 (3–10) | 7 (3–9) | 7 (3–10) | .503 |
| ICU admission | … | 123 (13.6%) | 75 (68.8%) | 48 (6%) | <.0001 |
| ICU admission within 48 h of hospital admission | … | 61 (6.7%) | 29 (26.6%) | 32 (4%) | <.0001 |
| PaO2/FiO2 | … | 224 (100–305) | 109 (100–229) | 238 (100–311) | <.0001 |
| >200 | 384 (42.5%) | 29 (26.6%) | 355 (44.7%) | <.0001 | |
| <200 | 311 (34.4%) | 63 (57.8%) | 248 (31.2%) | ||
| Unknown | 209 (23.1%) | 17 (15.6%) | 192 (24.2%) | ||
| Use of biological immunosuppressive drugs | … | 149 (16.5%) | 26 (23.9%) | 123 (15.5%) | .038 |
| Use of corticosteroids (available in n = 599) | … | 139 (23.2%) | 13 (21.3%) | 126 (23.4%) | .873 |
| Use of remdesivir | … | 32 (3.5%) | 2 (1.8%) | 30 (3.8%) | .414 |
| Use of heparin (available in n = 599) | … | 308 (51.4%) | 25 (40.9%)b | 283 (52.6%) | .085 |
| Hemoglobin, g/dL | … | 13.5 (12–14.7) | 13.8 (12–15) | 13.4 (12–14.6) | .300 |
| Platelets, per 109/L | … | 207 (155–272) | 220 (147–316) | 206 (156–269) | .331 |
| White blood cells, per 109/L | … | 6.9 (5–9.9) | 7.8 (5.4–12) | 6.8 (4.9–9.7) | .003 |
| Neutrophils, per 109/L | … | 4.9 (3.4–7.7) | 6 (4–9.7) | 4.9 (3.3–7.4) | .001 |
| Lymphocytes, per 109/L | … | 1 (0.7–1.3) | 0.8 (0.6–1.1) | 1 (0.7–1.4) | .017 |
| Creatinine, mg/dL | … | 0.98 (0.8–1.23) | 0.99 (0.85–1.28) | 0.97 (0.79–1.23) | .301 |
| ALT, U/L | … | 35 (23–56) | 41 (26–68) | 34 (22–55) | .010 |
| AST, U/L | … | 44 (31–66) | 49 (35–87) | 44 (30–63) | .003 |
| LDH, U/L | … | 363 (274–468) | 431 (339–597) | 355 (268–456) | <.0001 |
| Ferritin (available in n = 54), ng/mL | … | 926 (480–1684) | 1524 (1002–3097) | 888.5 (474–1499.5) | .134 |
| CRP, mg/L | … | 70.9 (29.1–132.5) | 117.2 (48.9–211.8) | 67.75 (26.9–123.7) | <.0001 |
| D-dimer (available in n = 204), μg/mL | … | 0.98 (0.53–2.46) | 1.32 (0.99–3.15) | 0.9 (0.5–2.37) | .010 |
| Prothrombin time, sec | … | 13.9 (13.1–15.3) | 14.2 (13.3–15.6) | 13.8 (13.1–15.3) | .265 |
| Cytolysis score | … | … | … | … | <.0001 |
| 0 | 527 (59.6%) | 54 (50.5%) | 473 (60.9%) | ||
| 1 | 157 (17.8%) | 14 (13.1%) | 143 (18.4%) | ||
| 2 | 127 (14.4%) | 15 (14%) | 112 (14.4%) | ||
| 3 | 73 (8.3%) | 24 (22.4%) | 49 (6.3%) | ||
| Inflammation score | … | … | … | … | <.0001 |
| 0 | 665 (75.5%) | 56 (53.3%) | 609 (78.5%) | ||
| 1 | 214 (24.3%) | 48 (45.7%) | 166 (21.4%) | ||
| 2 | 2 (0.2%) | 1 (1%) | 1 (0.1%) | ||
| Coagulation score | … | … | … | … | .191 |
| 0 | 554 (70.8%) | 68 (65.4%) | 486 (71.6%) | ||
| 1 | 209 (26.7%) | 31 (29.8%) | 178 (26.2%) | ||
| 2 | 20 (2.6%) | 5 (4.8%) | 15 (2.2%) |
Results are reported as median (IQR) or frequency (%). P-value < .05 are reported in bold. For variables with >10% missing values, actual numbers of observations are reported. Cytolysis score included alanine aminotransferase, aspartate aminotransferase, and lactate dehydrogenase. Inflammation score included ferritin and C-reactive protein. Coagulation score included D-dimer and prothrombin time. The 3 scores had a range of 0–2 or –3, with 0 corresponding to no abnormalities in inflammatory parameter levels and 2 or 3 corresponding to patients with markedly elevated values for all the considered laboratory parameters.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C-reactive protein; ICU, intensive care unit; IQR, interquartile range; LDH, lactate dehydrogenase; PaO2/FiO2, partial pressure of oxygen in the arterial blood/fraction of inspired oxygen.
By chi-square or Fisher's exact test (categorical variables) or Wilcoxon rank-sum test (continuous variables).
Twenty-four of 25 patients with secondary infections received heparin before the diagnosis of the infectious complication.
Thrombotic Complications
Thrombotic complications were documented in 72/904 patients (8.0%), who developed 87 events (9 SVT, 18 DVT, 5 AT, 55 PT), for an overall incidence rate of 4.94 (3.98–6.07) per 1000-PDFUs. Eleven out of 72 patients had thrombotic complications >48 hours after diagnosis of secondary infection. The 61/904 patients (6.7%) with a thrombotic complication diagnosed before or <48 hours after the diagnosis of a secondary infection developed 75 events (7 SVT, 17 DVT, 4 AT, 47 PT), with a median time since hospital admission (IQR) of 7 (2–13) days. Data regarding heparin use were available for 599 patients; among them, 308 (51.4%) received heparin. Characteristics of patients according to the diagnosis of thrombotic complications are detailed in Supplementary Table 1.
Secondary Infections
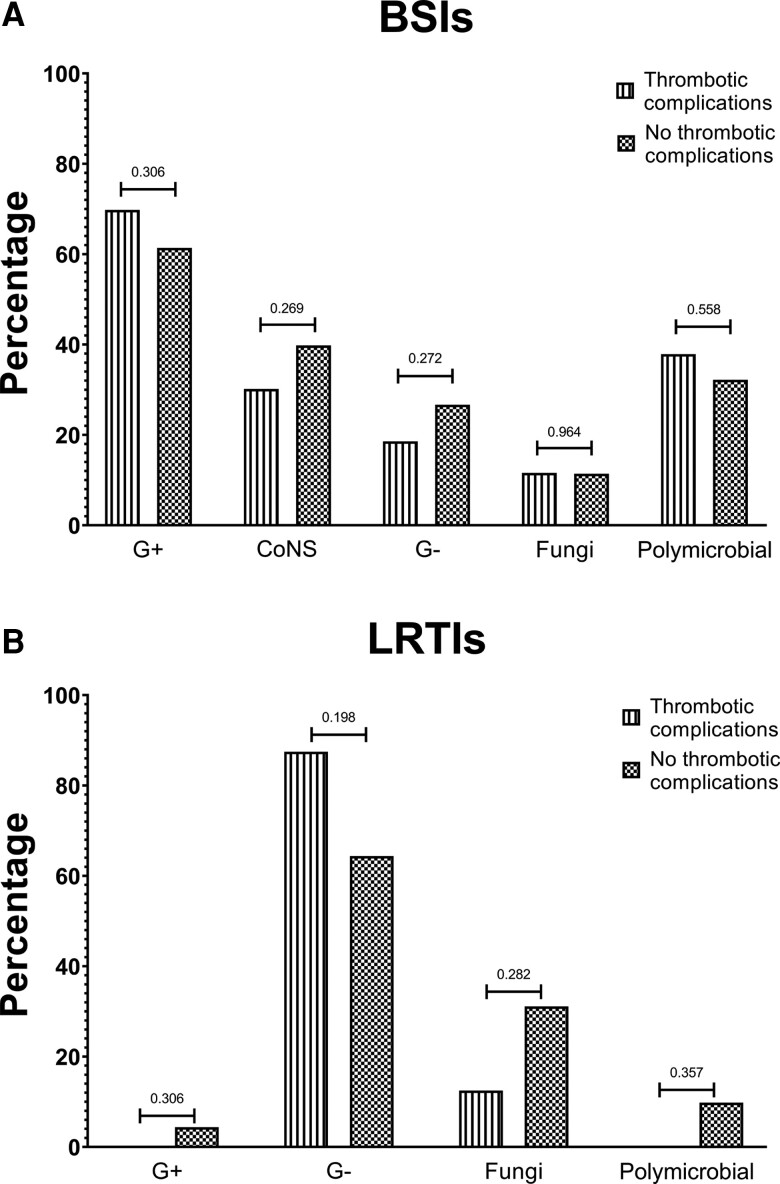
Secondary infections were documented in 109/904 patients (12.1%), who developed 176 events, with a median time since hospital admission (IQR) of 12 (8–18) days. Multiple secondary infections were diagnosed in 35 patients (3.9%). Characteristics of patients according to the diagnosis of secondary infections are shown in Table 1. The incidence rate of secondary infections was 10.0 (8.5–11.5) per 1000-PDFUs. Overall, 147 BSIs were documented in 97/904 patients (10.7%), while 48 LRTIs were diagnosed in 40 (4.4%) subjects, with a median time since hospital admission (IQR) of 12 (8–18) and 20 (9–34) days, respectively. The microbial etiology of BSIs and LRTIs is detailed in Supplementary Table 2. Among patients with BSIs, gram-positive microorganisms predominated (138/219 isolates, 63.0%), particularly coagulase-negative staphylococci (83/138, 60.1%), while gram-negative bacteria were 25.0% of the total (55/219). Multiple isolates were found in 49/147 events (33.3%). On the contrary, gram-negative microorganisms predominated among LRTIs (36/53 isolates, 67.9%), followed by Aspergillus spp. (15/53, 28.3%).
The distribution of microbial etiology of BSIs and LRTIs among patients with and without thrombotic complications was similar (Figure 1; Supplementary Tables 3 and 4), as was the frequency of polymicrobial infections.
Figure 1.
Microbial etiology of infectious complications. A, BSIs. B, LRTIs. Abbreviations: BSIs, bloodstream infections; CoNS, coagulase-negative staphylococci; G+, gram-positive; G-, gram-negative; LRTIs, lower respiratory tract infections.
Impact of Thrombotic Complications on the Development of Secondary Infections
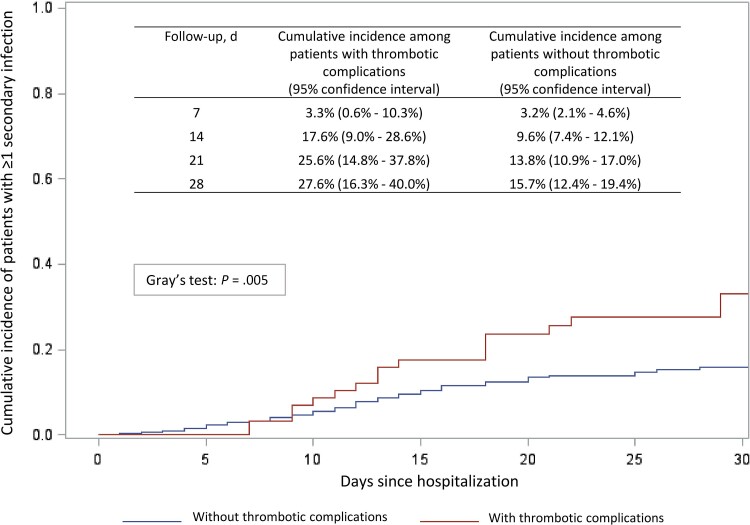
Secondary infections were observed at or after thrombotic complication diagnosis in 19/61 patients (31.1%), while 90/843 subjects (10.7%) without thrombotic complications developed a secondary infection (P < .0001). The incidence rate of secondary infections among patients with and without thrombotic complications was 15.0 (10.7–21.0) vs 9.3 (7.9–11.0) per 1000-PDFUs (P = .017). Specifically, the incidence rate of BSIs was 12.8 (8.9–18.4) vs 7.7 (6.4–9.2) per 1000-PDFUs (P = .021), while the incidence rate of LRTIs was 3.5 (1.5–7.0) vs 2.7 (1.9–3.6) per 1000-PDFUs (P = .469). Heparin use between patients with and without secondary infections was similar (40.9% vs 52.6%; P = .085); 24/25 patients with secondary infections received heparin before the diagnosis of the infectious complication.
The cumulative incidence function of secondary infections according to occurrence of thrombotic complications is shown in Figure 2.
Figure 2.
Cumulative incidence of patients with at least 1 secondary infection.
At multivariable analysis (Table 2), along with intensive care unit (ICU) admission within 48 hours from hospital admission and partial pressure of oxygen in the arterial blood/fraction of inspired oxygen, thrombotic complications were associated with development of secondary infections (subdistribution hazard ratio [sHR], 1.788; 95% CI, 1.018–3.140; P = .043). This finding was explored in a sensitivity analysis including use of heparin before diagnosis of secondary infections, where the association was confirmed, albeit without reaching statistical significance, possibly due to the limited number of included patients (sHR, 2.037; 95% CI, 0.979–4.238; P = .057) (Supplementary Table 5).
Table 2.
Multivariable Analysis on the Risk of Secondary Infections in Patients Hospitalized With Coronavirus Disease 19 (COVID-19)
| Characteristics (n = 745) | Subdistribution Hazard Ratio | 95% CI | P Valuea | ||
|---|---|---|---|---|---|
| Age, per 1 y older | 0.993 | 0.974 | 1.011 | .429 | |
| Sex, male vs female | 1.404 | 0.815 | 2.417 | .221 | |
| No. of comorbidities, per 1-point increase | 0.876 | 0.680 | 1.128 | .305 | |
| ICU admission within 48 h of hospital admission | 2.661 | 1.540 | 4.598 | .001 | |
| PaO2/FiO2 | >200 | … | … | … | Reference |
| <200 | 1.653 | 1.008 | 2.710 | .046 | |
| Unknown | 0.863 | 0.417 | 1.788 | .692 | |
| Use of biological immunosuppressive drugs, yes vs no | 1.141 | 0.687 | 1.894 | .611 | |
| Cytolysis score, per 1-point increase | 1.156 | 0.944 | 1.414 | .160 | |
| Inflammation score, per 1-point increase | 1.488 | 0.961 | 2.304 | .075 | |
| Coagulation score, per 1-point increase | 0.861 | 0.577 | 1.283 | .461 | |
| Thrombotic complications, yes vs no | 1.788 | 1.018 | 3.140 | .043 | |
P-value < .05 are reported in bold. Abbreviations: ICU, intensive care unit; PaO2/FiO2, partial pressure of oxygen in the arterial blood/fraction of inspired oxygen.
DISCUSSION
In our cohort, patients hospitalized with COVID-19 complicated by thrombotic events had a high risk of secondary infections.
Thrombotic complications were diagnosed in 8.0% of patients, with an overall incidence rate of 4.94 (3.98–6.07) per 1000-PDFUs; the majority of patients developed pulmonary thrombosis/thromboembolism. The incidence of thrombotic complications among patients with COVID-19 reported in the literature varies depending on the severity of illness, the use of prophylactic/therapeutic anticoagulation, and the presence of preexisting risk factors (up to 65% of non-ICU hospitalized patients and up to 85% of ICU patients in settings where systematic screening was applied) [22–25]. Indeed, the actual proportion of patients with thrombotic complications is likely higher than reported in the literature, as systematic screening for thrombosis has shown that the majority of patients do not have a suggestive clinical picture [26].
Secondary infections were documented in 12.1% of patients, with an incidence rate of 10.0 (8.5–11.5) per 1000-PDFUs. Our results are in line with available reports [9–11], highlighting the non-negligible proportion of hospitalized COVID-19 patients with infectious complications. The majority of patients had BSIs (10.0%), while LRTIs were diagnosed in 4.4% of subjects. Nevertheless, as only patients with microbiologically documented infections were considered in the analysis, the actual number of LRTIs would likely be higher than reported due to the limited number of patients who underwent invasive diagnostic procedures during the COVID-19 pandemic. Notably, microbial etiology was significantly different between patients with BSIs and LRTIs, with gram-positives as the main cause of secondary bloodstream infections and a prevalence of gram-negatives among pulmonary infections. As previously reported [19], COVID-19-associated pulmonary aspergillosis was documented in a significant proportion of patients, mainly in the ICU.
The incidence of secondary infections was significantly higher in patients with thrombotic complications (15.0 [10.7–21.0] vs 9.3 [7.9–11.0] per 1000-PDFUs; P = .017), especially when considering BSIs (12.8 [8.9–18.4] vs 7.7 [6.4–9.2] per 1000-PDFUs; P = .021). The association between thrombotic complications and secondary infections was confirmed at multivariable analysis, as patients with thrombotic events were shown to have about 1.8 times the risk of developing infectious complications compared with patients without thromboses (sHR, 1.788; 95% CI, 1.018–3.140; P = .043). Although patients with thrombotic events are likely to present with a severe or critical clinical picture, the finding of an independent association between thrombosis and secondary infections possibly reflects a shared pathophysiologic background. Indeed, the inflammation and cytolysis scores, both associated with secondary infections, were significantly higher in patients with thrombotic complications compared with those without. On the other hand, while the coagulation score was significantly higher in patients with thrombotic events, no significant differences were found between patients with and without secondary infections, suggesting that thrombotic events, irrespective of nonspecific alterations in the coagulation parameters, may be associated with subsequent infectious complications.
The high incidence of thrombotic events in patients with COVID-19 has already been linked to a series of processes collectively defined as immunothrombosis [1], but the pathogenic mechanisms underlying the possible relationship between thrombosis and predisposition to secondary infections have yet to be fully elucidated. Interestingly, along with the several mechanisms by which SARS-CoV-2 infection may lead to immune dysregulation (among others, T-cell functional exhaustion, inhibition of IFN signaling, and pathogen clearance due to phagocytes) [16], platelet activation during COVID-19 has been suggested to be associated with a state of immunosuppression [27]. It is possible to speculate that patients with a prominent immune activation leading to immunothrombosis may also be prone to paradoxical immunosuppression, leading to a heightened risk of secondary infections. In this context, more than a risk factor per se, thrombotic events may serve as a proxy of an impaired immune system. The finding of a similar microbial etiology of secondary infections in patients with and without thrombotic events may further support this hypothesis, as gram-positive pathogens (frequently seen in patients with septic thrombosis, especially if catheter-related [28]) were not predominant in patients with thrombosis.
The possible pathophysiological relationship between thrombosis and secondary infections in patients with COVID-19 seems attractive, as intervention aimed at dampening the immune dysregulation typical of SARS-CoV-2 infection may help reduce the incidence of thrombotic events and infectious complications. To our knowledge, there is a paucity of data regarding the impact of corticosteroids or other immune suppressors on development of thrombotic complications, while secondary infections do not seem to be more frequent in patients receiving these drugs [29]. This somewhat unexpected finding may indeed be related to the favorable impact of immune suppressors on COVID-19-associated immune dysregulation.
This study has some limitations. First, the study had a monocentric retrospective nature. Second, patients were enrolled during the first wave of the pandemic, and the clinical management of patients with COVID-19 changed thereafter with the widespread use of corticosteroids (and other immunosuppressors) and antithrombotic prophylaxis or treatment. Therefore, the results may not be generalizable to other epidemiological contexts. Third, a systematic assessment of infectious and thrombotic complications was not performed, possibly leading to underestimation of infectious and thrombotic events. Fourth, no data were available regarding the presence of intravascular catheters, which may have influenced the incidence of venous thrombosis. Finally, the main multivariable analysis did not include heparin use as a variable, given the reduced number of patients for whom this information was available; nevertheless, the sensitivity analysis including heparin confirmed the results of the main model.
In our cohort of COVID-19 hospitalized patients, thrombotic complications were associated with a high risk of secondary infections. The etiology of secondary infections was similar among patients with and without thrombotic complications.
Supplementary Material
Acknowledgments
We thank all the workers on the frontline during the COVID-19 pandemic.
Members of the COVID-BioB study group. Andolina Andrea, Bigoloni Alba, Bossolasco Simona, Bruzzesi Elena, Canetti Diana, Castiglioni Barbara, Cernuschi Massimo, Chiurlo Matteo, Cinque Paola, Dell’Acqua Raffaele, Della Torre Liviana, Gianotti Nicola, Guffanti Monica, Hasson Hamid, Messina Emanuela, Morsica Giulia, Nozza Silvia, Ranzenigo Martina, Uberti-Foppa Caterina, Vinci Concetta, Badalucco Ciotta Flavia, Bottanelli Martina, Clemente Tommaso, Mainardi Ilaria, Mori Giovanni, Papaioannu Borjesson Rebecka, Ponta Giacomo, Muccini Camilla, Mastrangelo Andrea, Oltolini Chiara, Spagnuolo Vincenzo, Benassi Luca, Bigai Giorgia, Bozzolo Enrica, Borio Giorgia, Bussolari Cecilia, Calvisi Stefania, Canti Valentina, Castellani Jacopo, Cavallo Ludovica, Cilla Marta, Cinel Elena, Compagnone Nicola, D’Aliberti Teresa, Damanti Sarah, De Lorenzo Rebecca, Di Lucca Giuseppe, Di Terlizzi Gaetano, Dumea Iulia, Farolfi Federica, Ferrante Marica, Frangi Claudia, Gallina Gabriele, Germinario Bruno Nicolò, Lanzillotta Marco, Li Voti Raffaele, Marinosci Alessandro, Martinenghi Sabina, Memoli Massimo, Montagna Marco, Pascali Maria, Patrizi Alessandro, Pomaranzi Chiara, Scotti Raffaella, Strada Silvia, Boffini Nicola, Cavalli Giulio, Della Torre Emanuel, De Luca Giacomo, Farina Nicola, Moroni Luca, Ramirez Giuseppe Alvise, Tomelleri Alessandro, Azzolini Maria Luisa, Baiardo Redaelli Martina, Calabrò Maria Grazia, Casiraghi Giuseppina Maria, Dell’Acqua Antonio, Fresilli Stefano, Guzzo Francesca, Landoni Giovanni, Lombardi Gaetano, Maimeri Nicolò, Moizo Elena, Nisi Francesco Giuseppe, Oriani Alessandro, Ortalda Alessandro, Pasculli Nicola, Pieri Marina, Turi Stefano, Bertoglio Luca, Bilman Victor, Carletti Silvia, Gona Floriana, Mancini Nicasio, Della Valle Patrizia, Molinari Chiara, Poloniato Antonella, Lalla Francesca, Prestifilippo Dario, Sapienza Jacopo, Seghi Federico.
Prior presentation. This work was previously presented during the 31st European Congress of Clinical Microbiology and Infectious Diseases (ECCMID; July 9, 2021; abstract number 989).
Contributor Information
Marco Ripa, Unit of Infectious and Tropical Diseases, IRCCS San Raffaele Scientific Institute, Milan, Italy; Vita-Salute San Raffaele University, Milan, Italy.
Laura Galli, Unit of Infectious and Tropical Diseases, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Armando D’Angelo, Coagulation Service and Thrombosis Research Unit, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Luca Apruzzi, Department of Vascular Surgery, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Diego Palumbo, Vita-Salute San Raffaele University, Milan, Italy; Unit of Radiology, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Corrado Campochiaro, Unit of Immunology, Rheumatology, Allergy and Rare Diseases, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Chiara Tassan Din, Unit of Infectious and Tropical Diseases, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Anna Danise, Unit of Infectious and Tropical Diseases, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Valentina Da Prat, General Medicine and Advanced Care Unit, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Giordano Vitali, Internal Medicine, Diabetes, and Endocrinology Unit, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Luigia Brugliera, Department of Rehabilitation and Functional Recovery, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Andrea Poli, Unit of Infectious and Tropical Diseases, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Roberta Monardo, Unit of Infectious and Tropical Diseases, IRCCS San Raffaele Scientific Institute, Milan, Italy; Vita-Salute San Raffaele University, Milan, Italy.
Giacomo Monti, Vita-Salute San Raffaele University, Milan, Italy; Anesthesia and Intensive Care Department, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Domenico Baccellieri, Department of Vascular Surgery, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Francesco De Cobelli, Vita-Salute San Raffaele University, Milan, Italy; Unit of Radiology, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Massimo Clementi, Vita-Salute San Raffaele University, Milan, Italy; Microbiology and Virology Department, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Sandro Iannaccone, Department of Rehabilitation and Functional Recovery, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Lorenzo Dagna, Vita-Salute San Raffaele University, Milan, Italy; Unit of Immunology, Rheumatology, Allergy and Rare Diseases, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Patrizia Rovere-Querini, Vita-Salute San Raffaele University, Milan, Italy; Internal Medicine, Diabetes, and Endocrinology Unit, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Fabio Ciceri, Vita-Salute San Raffaele University, Milan, Italy; Hematology and Bone Marrow Transplant Unit, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Moreno Tresoldi, General Medicine and Advanced Care Unit, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Alberto Zangrillo, Vita-Salute San Raffaele University, Milan, Italy; Anesthesia and Intensive Care Department, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Paolo Scarpellini, Unit of Infectious and Tropical Diseases, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Antonella Castagna, Unit of Infectious and Tropical Diseases, IRCCS San Raffaele Scientific Institute, Milan, Italy; Vita-Salute San Raffaele University, Milan, Italy.
COVID-BioB Study Group:
Andrea Andolina, Alba Bigoloni, Simona Bossolasco, Elena Bruzzesi, Diana Canetti, Barbara Castiglioni, Massimo Cernuschi, Matteo Chiurlo, Paola Cinque, Raffaele Dell’Acqua, Liviana Della Torre, Nicola Gianotti, Monica Guffanti, Hamid Hasson, Emanuela Messina, Giulia Morsica, Silvia Nozza, Martina Ranzenigo, Caterina Uberti-Foppa, Concetta Vinci, Flavia Badalucco Ciotta, Martina Bottanelli, Tommaso Clemente, Ilaria Mainardi, Giovanni Mori, Rebecka Papaioannu Borjesson, Giacomo Ponta, Camilla Muccini, Andrea Mastrangelo, Chiara Oltolini, Vincenzo Spagnuolo, Luca Benassi, Giorgia Bigai, Enrica Bozzolo, Giorgia Borio, Cecilia Bussolari, Stefania Calvisi, Valentina Canti, Jacopo Castellani, Ludovica Cavallo, Marta Cilla, Elena Cinel, Nicola Compagnone, Teresa D’Aliberti, Sarah Damanti, Rebecca De Lorenzo, Giuseppe Di Lucca, Gaetano Di Terlizzi, Iulia Dumea, Federica Farolfi, Marica Ferrante, Claudia Frangi, Gabriele Gallina, Nicolò Germinario Bruno, Marco Lanzillotta, Raffaele Li Voti, Alessandro Marinosci, Sabina Martinenghi, Massimo Memoli, Marco Montagna, Maria Pascali, Alessandro Patrizi, Chiara Pomaranzi, Raffaella Scotti, Silvia Strada, Nicola Boffini, Giulio Cavalli, Emanuel Della Torre, Giacomo De Luca, Nicola Farina, Luca Moroni, Alvise Ramirez Giuseppe, Alessandro Tomelleri, Luisa Azzolini Maria, Martina Baiardo Redaelli, Grazia Calabrò Maria, Maria Casiraghi Giuseppina, Antonio Dell’Acqua, Stefano Fresilli, Francesca Guzzo, Giovanni Landoni, Gaetano Lombardi, Nicolò Maimeri, Elena Moizo, Giuseppe Nisi Francesco, Alessandro Oriani, Alessandro Ortalda, Nicola Pasculli, Marina Pieri, Stefano Turi, Luca Bertoglio, Victor Bilman, Silvia Carletti, Floriana Gona, Nicasio Mancini, Patrizia Della Valle, Chiara Molinari, Antonella Poloniato, Francesca Lalla, Dario Prestifilippo, Jacopo Sapienza, and Federico Seghi
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Ortega-Paz L, Capodanno D, Montalescot G, et al. Coronavirus disease 2019–associated thrombosis and coagulopathy: review of the pathophysiological characteristics and implications for antithrombotic management. J Am Heart Assoc. 2021. 10.1161/JAHA.120.019650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jenner WJ, Kanji R, Mirsadraee S, et al. Thrombotic complications in 2928 patients with COVID-19 treated in intensive care: a systematic review. J Thromb Thrombolysis 2021; 51:595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Malas MB, Naazie IN, Elsayed N, Mathlouthi A, Marmor R, Clary B. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: a systematic review and meta-analysis. EClinicalMedicine 2020; 29–30:100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395:1033–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. The Lancet Haematology . COVID-19 coagulopathy: an evolving story. Lancet Haematol 2020; 7:e425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chan NC, Weitz JI. COVID-19 coagulopathy, thrombosis, and bleeding. Blood 2020; 136:381–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hunt BJ, De Paula EV, McLintock C, Dumantepe M. Prophylactic anticoagulation for patients in hospital with COVID-19. BMJ 2021; 372:n487. [DOI] [PubMed] [Google Scholar]
- 8. Piazza G, Morrow DA. Diagnosis, management, and pathophysiology of arterial and venous thrombosis in COVID-19. JAMA 2020; 324:2548–9. [DOI] [PubMed] [Google Scholar]
- 9. Grasselli G, Scaravilli V, Mangioni D, et al. Hospital-acquired infections in critically ill patients with COVID-19. Chest. 2021. 10.1016/j.chest.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Russell CD, Fairfield CJ, Drake TM, et al. Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: a multicentre, prospective cohort study. Lancet Microbe. 2021. 10.1016/S2666-5247(21)00090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Langford BJ, So M, Raybardhan S, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect 2020; 26:1622–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bhatt PJ, Shiau S, Brunetti L, et al. Risk factors and outcomes of hospitalized patients with severe coronavirus disease 2019 (COVID-19) and secondary bloodstream infections: a multicenter case-control study. Clin Infect Dis 2021; 72:e995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stephenson E, Reynolds G, Botting RA, et al. Single-cell multi-omics analysis of the immune response in COVID-19. Nat Med 2021; 27:904–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Files JK, Boppana S, Perez MD, et al. Sustained cellular immune dysregulation in individuals recovering from SARS-CoV-2 infection. J Clin Invest 2021; 131:e140491. 10.1172/JCI140491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang I, Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J Intensive Care. 2020. 10.1186/s40560-020-00453-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sopirala MM. Predisposition of COVID-19 patients to secondary infections: set in stone or subject to change? Curr Opin Infect Dis 2021; 34:357–64. [DOI] [PubMed] [Google Scholar]
- 17. Beristain-Covarrubias N, Perez-Toledo M, Thomas MR, Henderson IR, Watson SP, Cunningham AF. Understanding infection-induced thrombosis: lessons learned from animal models. Front Immunol 2019; 10:2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liesenborghs L, Meyers S, Vanassche T, Verhamme P. Coagulation: at the heart of infective endocarditis. J Thromb Haemost 2020; 18:995–1008. [DOI] [PubMed] [Google Scholar]
- 19. Koehler P, Bassetti M, Chakrabarti A, et al. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis 2021; 21:e149–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ripa M, Galli L, Poli A, et al. Secondary infections in patients hospitalized with COVID-19: incidence and predictive factors. Clin Microbiol Infect 2021; 27:451–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94:496–509. [Google Scholar]
- 22. Jenner WJ, Gorog DA. Incidence of thrombotic complications in COVID-19: on behalf of ICODE: The International COVID-19 Thrombosis Biomarkers Colloquium. J Thromb Thrombolysis. 2021. 10.1007/s11239-021-02475-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moores LK, Tritschler T, Brosnahan S, et al. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST guideline and expert panel report. Chest 2020; 158:1143–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baccellieri D, Bertoglio L, Apruzzi L, et al. Incidence of deep venous thrombosis in COVID-19 hospitalized patients during the first peak of the Italian outbreak. Phlebology 2021; 36:375–83. [DOI] [PubMed] [Google Scholar]
- 25. De Cobelli F, Palumbo D, Ciceri F, et al. Pulmonary vascular thrombosis in COVID-19 pneumonia. J Cardiothorac Vasc Anesth. 2021. 10.1053/j.jvca.2021.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pieralli F, Pomero F, Giampieri M, et al. Incidence of deep vein thrombosis through an ultrasound surveillance protocol in patients with COVID-19 pneumonia in non-ICU setting: a multicenter prospective study. PLoS One 2021; 16:e0251966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Feldman C, Anderson R. The role of co-infections and secondary infections in patients with COVID-19. Pneumonia (Nathan) 2021; 13:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 49:1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rochwerg B, Agarwal A, Siemieniuk RA, et al. A living WHO guideline on drugs for COVID-19. BMJ 2020; 370:m3379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.