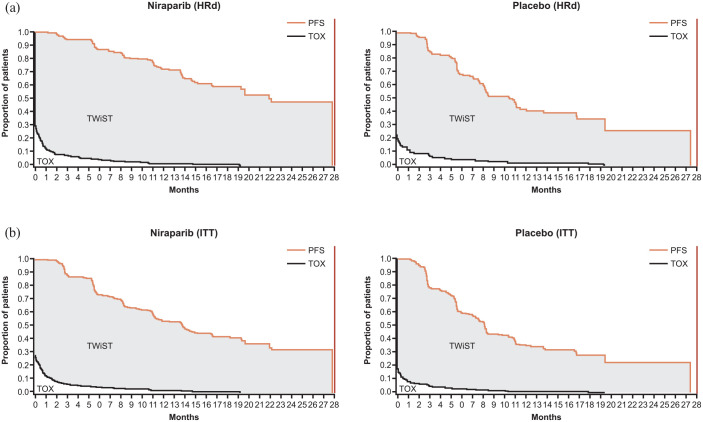
Figure 1.
Partitioned survival curves for the (a) HRd and (b) overall ITT populations.
TOX included grade ⩾2 AEs of interest (fatigue or asthenia, nausea, vomiting, abdominal pain, and abdominal bloating). HRd and overall ITT populations had a maximum PFS of 27.8 months.
AE, adverse event; HRd, homologous recombination deficient; ITT, intention-to-treat; PFS, progression-free survival; TOX, time before PFS during which patients experienced grade ⩾2 AEs; TWiST, time without symptoms of disease or toxicity.