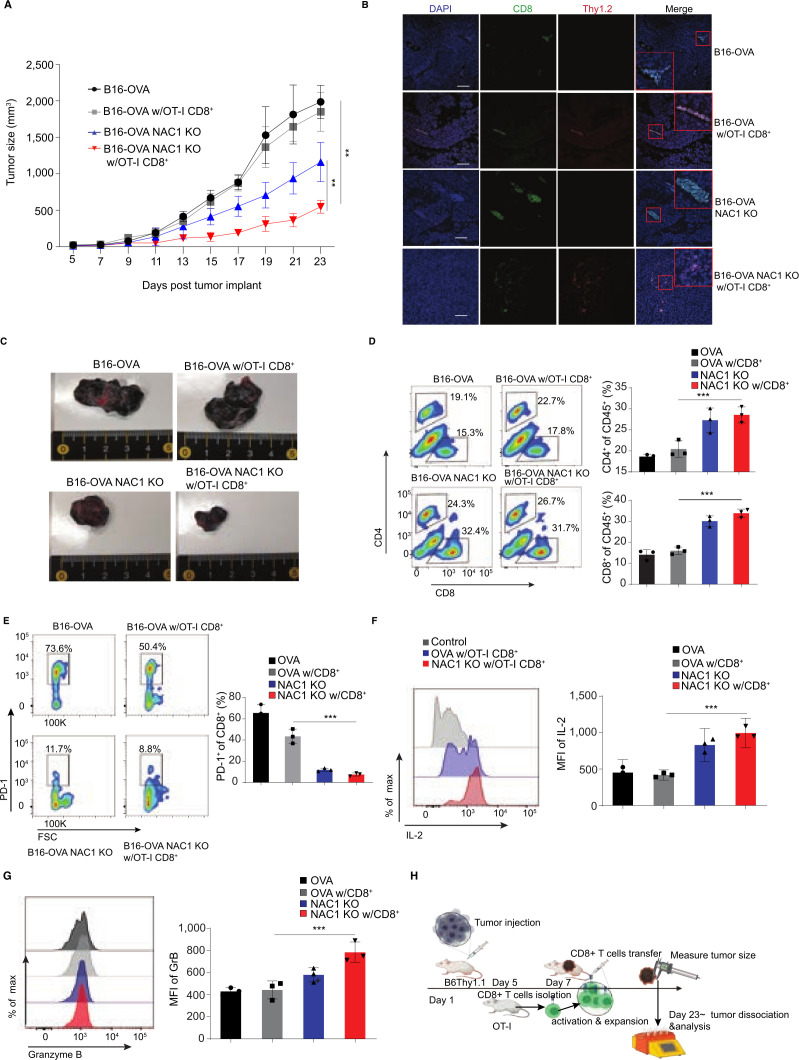
Figure 6.
NAC1 deficiency increases CTL infiltration in tumors following an ACT in immune-competent mice. Mouse WT B16-OVA or NAC1 KO B16-OVA tumor cells (1×106) were injected s.c. in the flank of B6. Thy1.1+ mice (n=5), followed by treatment with or without the ACT of OT-I CD8+ T cells. (A) Graphical representation of the progression of tumor size. (B) OT-I CD8+ T cell infiltration (Thy1.2+CD8+) in tumors was determined by immunofluorescence staining. (C) Indicated tumors were harvested and photographed on day 23. (D) Flow cytometric analysis of tumor-infiltrating CD4+ T cells and CD8+ T cells. (E) Histograms show expression of PD-1+ (left panel) and quantitation of PD-1+ cells of total infiltrated Thy1.2+ CD8+ cells (right panel). (F) Histograms showing production of IL-2 (left panel) and quantitation of MFI of IL-2 of total infiltrated CD8+ cells (right panel). (G) Histograms showing production of Granzyme B (left panel) and quantitation of MFI of Granzyme B of total infiltrated CD8+ cells (right panel). (H) Schematic representation of experimental paradigm. B6 Thy 1.1 mice were injected with mouse or human tumor cells on Day 1 followed by antigen specific CD8+ T cell transfer on day 7. The tumor size was monitored until day 23 post injection of the melanoma cells. All the results shown are representative of three identical experiments. *p≤0.05, ***p≤0.001. ACT, adoptive cell transfer; CTL, cytotoxic T lymphocytes; MFI, mean fluorescence intensity; NAC1, nucleus accumbens-associated protein-1; OVA, ovalbumin.