Abstract
Background
Improving dietary fat quality strongly affects serum cholesterol levels and hence the risk of cardiovascular diseases (CVDs). Recent studies have identified dietary fat as a potential modulator of the gut microbiota, a central regulator of host metabolism including lipid metabolism. We have previously shown a significant reduction in total cholesterol levels after replacing saturated fatty acids (SFAs) with polyunsaturated fatty acids (PUFAs). The aim of the present study was to investigate the effect of dietary fat quality on gut microbiota, short-chain fatty acids (SCFAs), and bile acids in healthy individuals. In addition, to investigate how changes in gut microbiota correlate with blood lipids, bile acids, and fatty acids.
Methods
Seventeen participants completed a randomized, controlled dietary crossover study. The participants received products with SFAs (control) or PUFAs in random order for three days. Fecal samples for gut microbiota analyses and fasting blood samples (lipids, fatty acids, and bile acids) were measured before and after the three-day intervention.
Results
Of a panel of 40 bacteria, Lachnospiraceae and Bifidobacterium spp. were significantly increased after intervention with PUFAs compared with SFAs. Interestingly, changes in Lachnospiraceae, as well as Phascolarlactobacterium sp. and Eubacterium hallii, was also found to be negatively correlated with changes in total cholesterol levels after replacing the intake of SFAs with PUFAs for three days. No significant differences in SCFAs or bile acids were found after the intervention.
Conclusion
Replacing SFAs with PUFAs increased the abundance of the gut microbiota family of Lachnospiraceae and Bifidobacterium spp. Furthermore, the reduction in total cholesterol after improving dietary fat quality correlated with changes in the gut microbiota family Lachnospiraceae. Future studies are needed to reveal whether Lachnospiraceae may be targeted to reduce total cholesterol levels.
Trial registration
The study was registered at Clinical Trials (https://clinicaltrials.gov/, registration identification number: NCT03658681).
Keywords: Dietary fat, Polyunsaturated fatty acids, Total cholesterol, Gut microbiota, Lachnospiraceae, Randomized controlled trial
Background
Improving dietary fat quality by exchanging the intake of saturated fatty acids (SFAs) with polyunsaturated fatty acids (PUFAs) has been shown to reduce serum cholesterol levels [1–4] and thereby the risk of cardiovascular diseases (CVDs) [3, 5]. Emerging evidence suggests that gut microbiota play a significant role in human metabolic regulation [6]. The gut microbiota and its host are in a symbiotic relationship by a joint utilization of consumed nutrients, and the human gut microbiota with its fermentation products are hypothesized to play a major role in host energy and substrate metabolism [7–9]. Hence, dietary habits affect both the abundance and composition of gut microbiota and host health [10–12]. Diet is one of the most important factors affecting the gut microbiota, and changes in diet can alter the gut microbiota within one to three days [13–16]. Recent studies have suggested that dietary fat is a potential modulator of the human gut microbiota composition [15, 17–20] and that both total dietary fat and fat quality may shape the gut microbiota [21]. The complex interplay between diet and the gut microbiota leads to the growth of specialist microbes that produce metabolites, such as short-chain fatty acids (SCFAs), influencing host metabolism and cholesterol regulation [17, 22–24]. The type of fat seems to elicit distinct effects on gut microbiota. While high-fat diets containing SFAs have been shown to decrease bacterial diversity [25], unsaturated fat is reported to increase the total bacterial count [26]. A correlation between omega-3 (n3) fatty acids and gut microbiota [27, 28], including a positive correlation with bacteria of the Lachnospiraceae family [28, 29], has been reported. In a recent study in mice, an omega-6 (n6) high-fat diet induced changes in gut microbiota, including a reduction in Lachnospiraceae [30]. However, only a few studies have investigated the effect of fat quality on gut microbiota and the impact on human metabolic regulation.
Bile acids are produced from cholesterol in the liver and facilitate digestion and absorption of lipids. Bile acids may also have hormonal or signaling functions by binding to several receptors and thereby play a crucial role in metabolic regulation [31]. It is well known that gut microbiota can oxidize and degrade bile acids. Even though gut microbiota may affect metabolic regulation by conversion of bile acids and the interaction between gut microbiota and bile acid metabolism is an emerging topic, there are few clinical studies investigating the interaction between gut microbiota, bile acid metabolism, and risk of CVD [32].
In a recent publication, it was reported that exchanging intake of SFAs with PUFAs effectively reduced total cholesterol levels after only three days in healthy individuals [1]. Whether this effect could be related to changes in the gut microbiota is not known.
The aim of the present study was to investigate the effect of dietary fat quality on gut microbiota, SCFAs, and bile acids in healthy individuals and to explore the relationship between gut bacteria and blood lipids, bile acids, and fatty acids.
Methods
Subjects and study design
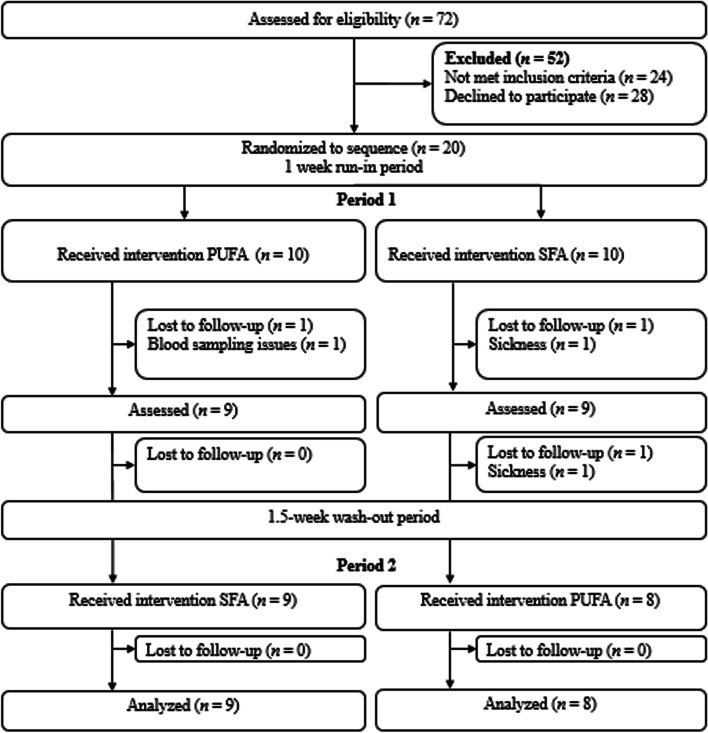
The results from the present study were obtained from the same participants and set of samples as previously described [1]. Healthy volunteers (aged 18–65 years) with body mass index (BMI) between 18.5–27 kg/m2 were recruited from advertisements on Facebook, Oslo Metropolitan University (OsloMet) website, and from the student mass and employees at OsloMet to participate in a randomized controlled crossover study as described previously [1]. The study was performed between April 2018 and January 2019 at OsloMet. Inclusion and exclusion criteria have been described previously [1]. Briefly, the exclusion criteria were fasting blood glucose values ≥ 6.1 mmol/L, any food allergies or intolerances or chronic metabolic diseases (e.g., diabetes, CVD, and cancer), including the use of medication, intestinal diseases, including inflammatory bowel disease, celiac disease, and irritable bowel disease. Furthermore, those with high sensitivity C-reactive protein (hsCRP) > 10 mg/L, treated with antibiotics the previous three months and during the study, donating blood the previous two months or during the study, were pregnant or lactating, planned weight reduction and/or had ≥ 5% weight change the previous three months, used any hormonal treatment (except from oral contraception), tobacco, or had a high alcohol consumption (> 40 g/day) were excluded. Seventy-two volunteers were screened for participation, and 20 participants were willing to participate in the dietary crossover study, while 17 completed the study. Participants lost during follow-up and those included in the study are outlined in the flow chart (Fig. 1).
Fig. 1.
Flow chart of the participants included in the dietary crossover study (n = 17) [1]. Abbreviations: n: number; PUFAs: polyunsaturated fatty acids; SFAs: saturated fatty acids
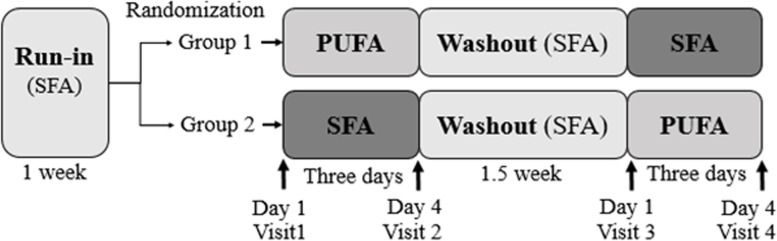
After a one-week run-in period, the participants were randomized (1:1 allocation ratio) and received either SFA products (muffins with butter-based spread and butter-based spread) (control) or PUFA products (muffins with margarine and margarine) for three consecutive days before crossing over to the other intervention, separated by a 1.5-week washout period (Fig. 2). In the run-in and washout periods, the participants received SFA (control) products. The study products (muffins and margarine or butter-based spread) replaced fat-containing products in their habitual diet. The fatty acid composition of the study products is given in Gaundal et al. [1]. The SFA products contributed 1085.5 kcal, 78.5 g carbohydrate, 13.4 g protein, 79.5 g total fat, 29.9 g SFAs, 33.4 g monounsaturated fatty acids (MUFAs) and 10.2 g PUFAs per day. The PUFA products contributed 985.8 kcal, 74.4 g carbohydrate, 13.9 g protein, 69.8 g total fat, 2.4 g SFAs, 30.9 g MUFAs and 26.4 g PUFAs per day [1].
Fig. 2.
Study design of the double-blind, randomized, controlled crossover study. Described previously [1]. Seventeen healthy volunteers (group 1: n = 9; group 2: n = 8) received daily PUFA products (two muffins and 20 g margarine spread) or SFA products (two muffins and 20 g butter-based spread) for three consecutive days, separated by a 1.5-week washout period. The participants received SFA products in the run-in and washout periods. Fasted blood was measured for glucose, insulin, triglyceride, NEFAs, fatty acid profiles, SCFAs, bile acids, and total cholesterol at each visit. In addition, fecal samples were collected for gut microbiota analyses at each visit. Abbreviations: NEFAs: nonesterified fatty acids, PUFAs: polyunsaturated fatty acids, SCFAs: short-chain fatty acids, SFAs: saturated fatty acids
The participants were advised to maintain their habitual diet and physical activity level throughout the study period, except for the dietary restrictions: limit their intake of dietary fats and products rich in beta-glucan, not taking dietary supplements (fish oil, probiotics, etc.) and antibiotics. Details about the study design, description of participants, and study products are described elsewhere [1].
All participants signed a written informed consent form prior to participation. The study was approved by the Regional Committees for Medical and Health Research Ethics (2018/104) and conducted according to the Declaration of Helsinki guidelines. The crossover study was registered at Clinical Trials (http://clinicaltrials.gov/, registration identification number: NCT03658681).
Blood sampling and laboratory analyses
Blood samples were collected after an overnight fast (≥ 12 h) at each visit in the crossover study [1]. Fasting triglyceride and total cholesterol were measured in serum by a routine laboratory (Fürst Medical Laboratory) within 24 h. Furthermore, nonesterified fatty acids (NEFAs), fatty acid profiles and the SCFAs acetate, propionate and butyrate were measured in EDTA plasma in a commercial laboratory (Vitas Analytical Service, Oslo, Norway). NEFAs were measured using an enzymatic colorimetric assay with acyl-CoA oxidase and MEHA as color reagents. The total plasma (EDTA) fatty acid profile was measured with GC flame ionization detection. An internal standard (triheptadecanoin) was added, and the samples were methylated with 3 N HCl in methanol. Fatty acid methyl esters were extracted with hexane, and then samples were neutralized with 3 N KOH in water. After mixing and centrifuging, the hexane phase was injected into the GC flame ionizing detection. Analysis was performed on a 7890A GC with a split/splitless injector, a 7683B automatic liquid sampler and flame ionization detection (Agilent Technologies). Separations were performed on an SP-2380 (30 m × 0·25 mm i.d. × 0·25 μm film thickness) column from Supelco. The concentration of the individual fatty acids was measured as μg fatty acid/ml plasma and presented as a percentage of total fatty acids.
Nine bile acids, cholic acid (CA), deoxycholic acid (DCA), chenodeoxycholic acid (CDCA), taurocholic acid (TCA), taurodeoxycholic acid (TDCA), taurochenodeoxycholic acid (TCDCA), glycocholic acid (GCA), glycodeoxycholic acid (GDCA), and glycochenodeoxycholic acid (GCDCA), were measured in plasma using LC‒MS/MS with a sample volume of 50 µL and a single deuterium-labeled isotope as an internal standard. The plasma was initially treated with aqueous formic acid [1%] prior to protein precipitation with acetonitrile. The extracts were loaded onto a Hybrid SPE Phospholipid 96-well plate, and the effluent was dried in a vacuum centrifuge concentrator. LC‒MS/MS was performed on the reconstituted residues using a Shimadzu LC-20ADXR LC system coupled to a Sciex QTRAP5500 mass spectrometer with a Turbo V ion source and a TurboIonspray probe. Separation of the analytes was achieved using a Phenomenex Kinetex 2.6 μm Biphenyl 100A 100 × 4.6 mm column with an aqueous solution of ammonium acetate [5 mM] and acetic acid [2.1 mM] and an acetonitrile gradient mobile phase. Negative mode multiple reaction monitoring was used for detection. Linear calibration curves of the peak area ratios of the analyte and internal standard in aqueous methanol [50%] were used for quantification.
Fecal collection and gut microbiota analyses
The participants received a sample collection kit (GA-map™ Dysbiosis Test, Genetic Analysis AS) for fecal collection and were instructed to sample the stool from three different places and place it in the sampling tubes. The samples were kept at room temperature for a maximum of three days before they were stored at -80 °C at OsloMet. The participants were instructed to sample the stool before (day 1) and after (day 4) each intervention, as close to the visits as possible. Fecal samples were collected at each visit. All samples were collectively sent to Genetic Analysis AS (GA) for microbiota analyses after the study was completed. The GA-map™ Dysbiosis Test is a commercially available genome-based test using fecal samples for analyses of gut bacteria, described in detail elsewhere [33]. The test comprises 48 DNA probes targeting ≥ 300 bacteria on different taxonomic levels, thus allowing mapping of the intestinal microbiota profile for a selected set of bacteria. To characterize and identify bacteria present, probes targeting seven variable regions (V3—V9) of the 16S rRNA gene were used. Human fecal sample homogenization and mechanical and enzymatic bacterial cell disruption were utilized to isolate and bind total bacterial genomic DNA to magnetic beads. The hypervariable regions V3—V9 of the 16S rRNA were further amplified by polymerase chain reaction (PCR). To determine bacterial DNA labeling, single nucleotide extension and hybridization to a complementary DNA strand were coupled to beads. The abundance of bacteria was assessed by the strength of the fluorescent signal (probe intensity) and measured by a Luminex 200 (Luminex Corporation). Of the 48 bacteria, 40 were detected in more than 50% of the participants at each timepoint and included in the analyses.
Statistical analyses
The primary aim of the crossover study was to investigate the effect of fat quality on glycemic regulation, and the sample size was based on literature on fat and glycemic regulation as described previously [1], whereas secondary outcomes were fasting blood lipids, fatty acids, SCFAs, bile acids, and gut microbiota. Hence, the nature of the present study is explorative, and therefore correction for multiple testing has not been performed.
For the gut microbiota analyses, the relative abundance number was logarithmically transformed with base 2 (Log2) to reduce the skewness of the original data. The log2 ratio was obtained by calculating the ratio before and after intervention with SFAs or PUFAs. A paired t test was used to assess differences between the two interventions based on the log2 ratio. In addition, a paired t test was also used to calculate differences within each group (SFAs and PUFAs) based on the log2 values before and after intake of SFAs and PUFAs.
For the variables of SCFAs and bile acids, a paired sample comparison (Wilcoxon signed rank test) was used to assess differences between the two interventions (SFA and PUFA) and differences within each group. SCFA levels were reported as both absolute values and as a percent of the total SCFA level.
Associations between the change in blood parameters (lipids, fatty acids, bile acids) and the change in gut microbiota were assessed with Pearson correlation. P < 0.05 was regarded as statistically significant for all statistical analyses. Statistical analyses were performed with IBM SPSS statistics (version 27), and figures were designed with GraphPad Prism 8 for Windows (version 8.0.0.).
Results
The participants were healthy and normal weight, with fasting glucose, hemoglobin A1c (HbA1c), triglyceride, total cholesterol, and diastolic and systolic blood pressure within the normal range (Table 1). Their baseline characteristics have previously been shown [1].
Table 1.
Baseline characteristics of the participants, as previously shown [1]
| Median | 25th—75th percentiles | ||
|---|---|---|---|
| Male/female (n) | 6/11 | ||
| Age (years) | 28.0 | 25.0—46.0 | |
| BMI (kg/m2) | 22.8 | 22.0—25.0 | |
| HbA1c (%)a | 5.2 | 5.0—5.4 | |
| HbA1c (mmol/mol)a | 33.0 | 31.2—35.5 | |
| Glucose (mmol/L) | 5.1 | 4.9—5.4 | |
| Insulin (pmol/L) | 51.0 | 31.0—60.0 | |
| Triglyceride (mmol/L) | 0.9 | 0.6—1.4 | |
| Total cholesterol (mmol/L) | 4.9 | 4.4—5.4 | |
| hsCRP (mg/L) | 0.9 | 0.3—1.4 | |
| Systolic blood pressure (mmHg)a | 123 | 113—136 | |
| Diastolic blood pressure (mmHg)a | 71 | 66—75 | |
| Body fat percent (%) | 24.4 | 12.5—33.9 | |
| FFM (kg) | 47.8 | 45.3—69.3 |
aHbA1c, systolic and diastolic blood pressure measured at screening. Variables are measured fasted
FFM Fat free mass, HbA1c Hemoglobin A1c, hsCRP High sensitivity C-reactive protein
Whether intake of different fat qualities could affect the gut microbiota was further addressed. Of the 40 gut bacteria analysed, the abundance of Bifidobacterium spp. and Lachnospiraceae was significantly increased after intake of PUFAs compared to SFAs (P = 0.029 and P = 0.013, respectively) (Table 2). Furthermore, there was a tendency toward differences in the abundance of Actinobacteria, Dialister invisus, Dialister invisus & Megasphaera micronuciformis, Eubacterium hallii and Phascolactobacterium sp. between the interventions (Table 2).
Table 2.
Differences in bacterial abundance after a three-day intervention with SFA and PUFA
| SFA | PUFA | ||||||
|---|---|---|---|---|---|---|---|
| Gut bacteria | Day 1 | Day 4 | p1 | Day 1 | Day 4 | p1 | p2 |
| Actinobacteria (A) | 7.9 (1.1) | 7.4 (1.1) | 0.087 | 7.5 (1.2) | 7.7 (1.3) | ns | 0.063 |
| Actinomycetales (A) | 4.0 (1.1) | 4.0 (1.0) | ns | 4.4 (1.3) | 4.2 (1.0) | ns | ns |
| Bifidobacterium spp. (A) | 7.1 (1.3) | 6.6 (1.3) | 0.091 | 6.6 (1.3) | 7.0 (1.4) | ns | 0.029 |
| Alistipes (B) | 7.1 (1.8) | 7.3 (1.8) | ns | 7.2 (1.8) | 7.5 (1.8) | 0.006 | ns |
| Alistipes onderdonkii (B) | 4.2 (1.9) | 4.3 (1.9) | ns | 4.2 (1.9) | 4.5 (2.1) | ns | ns |
| Bacteroides fragilis (B) | 4.0 (2.0) | 4.2 (2.1) | ns | 3.8 (1.8) | 4.1 (2.2) | ns | ns |
| Bacteroides pectinophilus (B) | 3.6 (0.6) | 3.5 (0.5) | ns | 3.7 (0.7) | 3.6 (0.7) | ns | ns |
| Bacteroides spp. (B) | 5.3 (1.6) | 5.4 (1.6) | ns | 5.3 (1.5) | 5.4 (1.5) | ns | ns |
| Bacteroides spp. & Prevotella spp. (B) | 9.9 (0.4) | 10.0 (0.3) | ns | 9.9 (0.4) | 9.9 (0.3) | ns | ns |
| Bacteroides stercoris (B) | 3.8 (1.7) | 3.9 (1.5) | ns | 3.8 (1.5) | 3.8 (1.4) | ns | ns |
| Bacteroides zoogleoformans (B) | 3.7 (1.5) | 4.1 (1.6) | 0.086 | 3.9 (1.5) | 3.9 (1.4) | ns | ns |
| Parabacteroides johnsonii (B) | 3.9 (1.8) | 4.0 (1.6) | ns | 3.9 (1.8) | 4.3 (1.8) | 0.014 | ns |
| Parabacteroides spp. (B) | 5.5 (1.5) | 5.6 (1.5) | ns | 5.5 (1.5) | 5.8 (1.6) | ns | ns |
| Bacilli (F) | 7.2 (0.4) | 7.1 (0.7) | ns | 7.1 (0.8) | 7.1 (0.5) | ns | ns |
| Clostridia (F) | 8.9 (0.2) | 9.0 (0.3) | ns | 8.9 (0.3) | 9.0 (0.2) | ns | ns |
| Clostridium methylpentosum (F) | 4.1 (0.8) | 4.2 (0.8) | ns | 4.1 (0.8) | 4.4 (0.9) | 0.082 | ns |
| Coprobacillus cateniformis (F) | 2.9 (0.9) | 2.8 (0.5) | ns | 3.0 (1.0) | 2.9 (1.2) | ns | ns |
| Dialister invisus (F) | 7.1 (2.3) | 7.3 (2.4) | ns | 7.5 (2.3) | 7.3 (2.2) | ns | 0.073 |
| Dialister invisus & Megasphaera micronuciformis (F) | 5.6 (2.6) | 5.8 (2.6) | 0.005 | 5.9 (2.8) | 5.8 (2.6) | ns | 0.067 |
| Dorea spp. (F) | 4.5 (1.0) | 4.5 (0.9) | ns | 4.5 (1.2) | 4.7 (0.9) | ns | ns |
| Eubacterium hallii (F) | 6.6 (0.8) | 6.3 (0.9) | 0.064 | 6.3 (0.7) | 6.5 (0.9) | ns | 0.095 |
| Eubacterium biforme (F) | 5.0 (2.4) | 4.9 (2.2) | ns | 4.8 (2.2) | 4.7 (2.2) | ns | ns |
| Eubacterium rectale (F) | 6.2 (2.2) | 6.2 (2.1) | ns | 6.2 (2.0) | 6.4 (2.0) | ns | ns |
| Eubacterium siraeum (F) | 4.4 (1.1) | 4.1 (1.1) | 0.043 | 4.7 (0.8) | 4.7 (1.2) | ns | ns |
| Firmicutes (F) | 9.4 (0.4) | 9.4 (0.6) | ns | 9.5 (0.5) | 9.4 (0.5) | ns | ns |
| Faecalibacterium prausnitzii (F) | 8.8 (0.3) | 8.7 (0.5) | ns | 8.7 (0.3) | 8.9 (0.3) | ns | ns |
| Lachnospiraceae (F) | 9.8 (0.2) | 9.8 (0.2) | ns | 9.7 (0.3) | 9.8 (0.2) | 0.009 | 0.013 |
| Lactobacillus spp. (F) | 5.7 (2.3) | 5.7 (2.0) | ns | 5.5 (2.1) | 5.5 (2.0) | ns | ns |
| Lactobacillus spp. 2 (F) | 3.5 (1.0) | 3.5 (1.2) | ns | 3.2 (1.1) | 3.4 (1.3) | ns | ns |
| Phascolarctobacterium sp. (F) | 4.2 (1.9) | 4.2 (1.8) | ns | 4.1 (1.8) | 4.2 (1.9) | 0.082 | 0.069 |
| Ruminococcus albus & Ruminococcus bromii (F) | 4.9 (1.9) | 5.0 (2.0) | ns | 5.1 (1.9) | 5.4 (2.0) | ns | ns |
| Ruminococcus gnavus (F) | 4.0 (1.2) | 4.0 (1.3) | ns | 3.9 (1.1) | 4.0 (1.1) | ns | ns |
| Streptococcus agalactiae & Eubacterium rectale (F) | 5.9 (1.7) | 5.9 (1.6) | ns | 5.7 (1.7) | 5.9 (1.5) | 0.065 | ns |
| Streptococcus salivarius ssp. thermophilus & S. sanguinis (F) | 2.8 (0.4) | 2.8 (0.5) | ns | 3.4 (1.6) | 3.3 (1.1) | ns | ns |
| Streptococcus salivarius ssp. thermophilus (F) | 4.6 (1.4) | 4.6 (1.4) | ns | 4.7 (1.6) | 4.8 (1.5) | ns | ns |
| Streptococcus spp. 2 (F) | 3.5 (0.9) | 3.4 (1.0) | ns | 3.3 (0.8) | 3.3 (0.8) | ns | ns |
| Veillonella spp. (F) | 9.2 (0.4) | 9.2 (0.4) | ns | 9.2 (0.5) | 9.2 (0.4) | ns | ns |
| Firmicutes various (F/T/B) | 7.7 (1.5) | 7.5 (1.4) | ns | 7.4 (1.5) | 7.6 (1.5) | ns | ns |
| Proteobacteria (P) | 5.9 (0.3) | 6.0 (0.8) | ns | 6.0 (0.5) | 6.0 (0.5) | ns | ns |
| Mycoplasma hominis (T) | 6.3 (2.3) | 6.3 (2.2) | ns | 6.1 (2.2) | 5.9 (2.3) | ns | ns |
The values are log2 transformed and given as mean (SD). P-values indicate differences within (p1) or between (p2) SFA and PUFA interventions calculated with t-test
A Actinobacteria, B Bacteroidetes, F Firmicutes, ns Non-significant, P Proteobacteria, PUFA Polyunsaturated fatty acids, SFA Saturated fatty acids, T Tenericutes
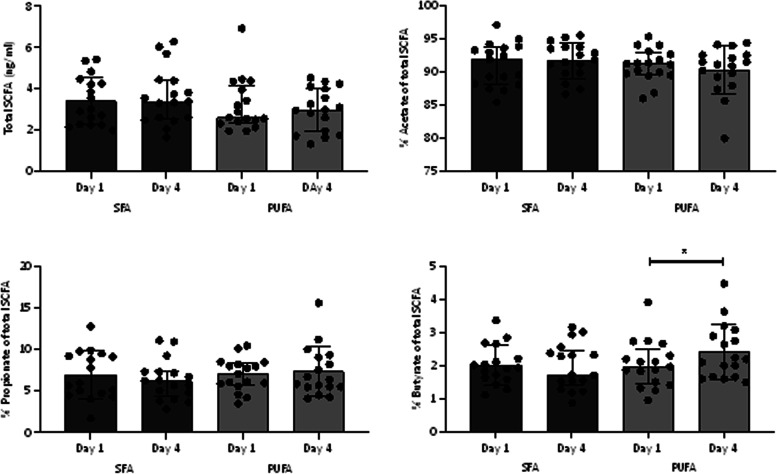
The microbial metabolites SCFAs acetate, propionate and butyrate were measured in blood before and after intake of SFAs and PUFAs. There were no significant differences in total SCFA levels or relative levels of acetate, propionate, or butyrate between the interventions (Fig. 3). However, intake of PUFAs for three consecutive days significantly increased the relative level of butyrate (P = 0.015) (Fig. 3).
Fig. 3.
SCFAs before and after intake of SFAs or PUFAs. Total SCFAs and relative levels of acetate, propionate, and butyrate before (day 1) and after (day 4) intake of SFAs and PUFAs in healthy individuals (n = 17) for three consecutive days. Data are shown as the median, and bars indicate the 25th—75th percentiles. Within- and between-group differences were analyzed with the Wilcoxon signed rank test. * indicates P ≤ 0.05. Abbreviations: PUFAs: polyunsaturated fatty acids, SCFAs: short-chain fatty acids, SFAs: saturated fatty acids
We have previously shown that total cholesterol levels significantly decreased after intervention with PUFAs compared with SFAs, whereas no significant effect on triglycerides and NEFAs was found [1]. As bile acids are synthesized from cholesterol, we wanted to investigate the effect of dietary fat on circulating levels of bile acids. Of the nine bile acids measured in plasma, no significant changes between the interventions were detected. Within interventions, however, changes were evident. Within the SFA intervention, TCA, GCA, and GCDCA decreased significantly (P = 0.039, P = 0.013, P = 0.028, respectively), while GDCA decreased and GCDCA increased within the PUFA intervention (P = 0.017, P = 0.025, respectively) (Table 3).
Table 3.
Differences in bile acids after a three-day intervention with SFA and PUFA
| SFA | PUFA | ||||||
|---|---|---|---|---|---|---|---|
| Bile acid (nM) | Day 1 | Day 4 | p1 | Day 1 | Day 4 | p1 | p2 |
| Cholic acid (CA) | 27.6 (20.2–111.2) | 26.2 (12.2–299.0) | 0.723 | 84.5 (14.2–239.4) | 24.0 (8.7–83.5) | 0.193 | 0.102 |
| Deoxycholic acid (DCA) | 244.9 (117.5–496.6) | 275.0 (143.2–404.8) | 0.332 | 253.0 (170.2–539.0) | 207.7 (161.8–311.6) | 0.113 | 0.124 |
| Chenodeoxycholic acid (CDCA) | 116.0 (61.9–194.9) | 65.0 (35.9–284.6) | 0.653 | 135.9 (65.3–315.7) | 82.7 (17.0–216.4) | 0.193 | 0.068 |
| Taurocholic acid (TCA) | 3.6 (1.5–7.8) | 2.3 (0.6–4.1) | 0.039 | 3.4 (1.9–14.5) | 2.7 (1.4–8.3) | 0.237 | 0.868 |
| Taurodeoxycholic acid (TDCA) | 26.9 (11.2–46.6) | 18.9 (6.5–38.7) | 0.177 | 25.6 (11.4–72.0) | 17.7 (14.0–33.7) | 0.193 | 0.586 |
| Taurochenodeoxycholic acid (TCDCA) | 46.6 (24.6–99.7) | 43.1 (18.1–64.9) | 0.062 | 60.3 (34.2–101.3) | 54.6 (19.5–90.2) | 0.163 | 0.831 |
| Glycocholic acid (GCA) | 47.4 (33.2–99.0) | 32.3 (17.2–72.1) | 0.013 | 58.3 (27.6–112.3) | 53.8 (15.7–90.1) | 0.287 | 0.869 |
| Glycodeoxycholic acid (GDCA) | 153.4 (76.4–236.8) | 99.0 (50.5–205.8) | 0.124 | 162.0 (74.2–285.3) | 121.6 (62.9–165.5) | 0.017 | 0.210 |
| Glycochenodeoxycholic acid (GCDCA) | 549.2 (219.6–724.7) | 300.6 (222.3–491.1) | 0.028 | 442.4 (248.6–763.0) | 443.8 (108.8–585.9) | 0.025 | 0.653 |
Data is given as median (25th—75th percentiles). P-values indicate differences within (p1) or between (p2) SFA and PUFA interventions calculated with Wilcoxon signed rank test
nM Nanomolar, PUFA Polyunsaturated fatty acids, SFA Saturated fatty acids
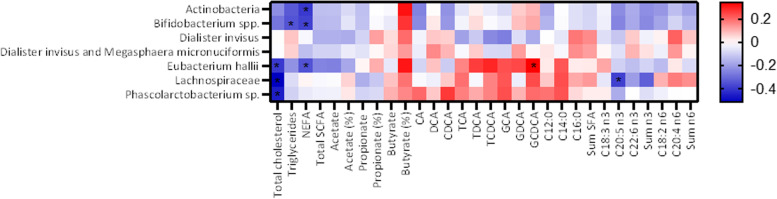
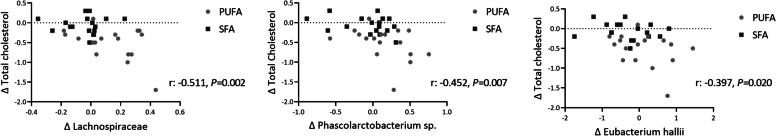
The gut bacteria that significantly differed or tended to differ after intervention with SFAs compared to PUFAs were thereafter correlated with the other variables. Changes in gut microbiota were associated with changes in total cholesterol levels, triglycerides, bile acids, and fatty acids (Fig. 4). We found a significant, negative correlation between the change in total cholesterol and the change in abundance of Lachnospiraceae (r: -0.511, P = 0.002), Phascolarctobacterium sp. (r: -0.452, P = 0.007), and Eubacterium hallii (r: -0.397, P = 0.020) (Figs. 4 and 5). There were also significant negative correlations between changes in NEFAs and changes in the abundance of Actinobacteria (r: -0.397, P = 0.020), Eubacterium hallii (r: -0.348, P = 0.044), and Bifidobacterium spp. (r: -0.379, P = 0.027) and between changes in triglycerides and Bifidobacterium spp. (r: -0.346, P = 0.045) (Fig. 4). Interestingly, we found that three bacteria (Eubacterium hallii, Lachnospiraceae, and Phascolarctobacterium sp.) correlated negatively with total cholesterol and positively with several of the bile acids. However, a significant positive correlation was only found between changes in the bile acid GCDCA and Eubacterium hallii (r: 0.343, P = 0.047) (Fig. 4). We also investigated correlations between the gut bacteria and fatty acids in blood. The change in Lachnospiraceae after intervention was negatively correlated with the change in eicosapentaenoic acid (EPA) (C20:5 n3) (r: -0.384, P = 0.025), but no other significant correlations were observed between the gut bacteria and fatty acids in blood (Fig. 4). Correlations between gender and age and the different variables were investigated. Age correlated significantly with Eubacterium hallii (r: 0.353, P = 0.040), Eubacterium biforme (r: 0.364, P = 0.034), Eubacterium rectale (r: -0.361, P = 0.036) and 20:4 n6 (r: 0.349, P = 0.043), while sex correlated significantly with Bacilli (r: -0.348, P = 0.043), Actinomycetales (r: -0.390, P = 0.022), Shigella spp. & Echerichia spp. (r: -0.346, P = 0.045), butyrate (r: 0.357, P = 0.038), and the bile acids GCA (r: -0.409, P = 0.016) and GCDCA (r: -0.408, P = 0.017).
Fig. 4.
Relationship between changes in gut bacteria and total cholesterol, triglycerides, NEFAs, SCFAs, bile acids, and fatty acid profiles after intervention with SFAs and PUFAs. Correlation analysis was performed with Pearson correlation. * indicates P ≤ 0.05. Abbreviations: C12:0: lauric acid, C14:0: myristic acid, C16:0: palmitic acid, C18:3 n3: alpha-linolenic acid, C20:5 n3: eicosapentaenoic acid (EPA), C22:6 n3: docosahexaenoic acid (DHA), C18:2 n6: linoleic acid, C20:4 n6: arachidonic acid, CA: cholic acid, CDCA: chenodeoxycholic acid, DCA: deoxycholic acid, GCA: glycocholic acid, GDCA: glycodeoxycholic acid, GCDCA: glycochenodeoxycholic acid, n3: omega-3, n6: omega-6, NEFAs: nonesterified fatty acids, PUFAs: polyunsaturated fatty acids, SCFAs: short-chain fatty acids, SFAs: short-chain fatty acids, TCA: taurocholic acid, TDCA: taurodeoxycholic acid, TCDCA: taurochenodeoxycholic acid
Fig. 5.
Relationship between the change in abundance of bacteria and total cholesterol level after interventions with SFAs and PUFAs. Correlation analysis was performed with Pearson correlation, and * indicates P ≤ 0.05. Abbreviations: PUFAs: polyunsaturated fatty acids, SFAs: saturated fatty acids
Discussion
In this study, the effect of different fat qualities (SFAs vs. PUFAs) on the human gut microbiota, SCFAs, and bile acids in healthy individuals was examined, in addition to the relationship between changes in gut bacteria and blood total cholesterol, triglycerides, NEFAs, bile acids, and fatty acid profiles. In a recent study, Gaundal et al. showed that replacing the intake of SFAs with PUFAs reduced total cholesterol levels by 8% after only three days in healthy individuals [1]. Accompanied by the reduced total cholesterol level, the present study demonstrates a shift in specific gut bacteria after replacing intake of SFAs with PUFAs for three days and identified specific bacteria associated with cholesterol levels in healthy individuals.
The abundance of Lachnospiraceae and Bifidobacterium spp. increased after three days following intake of PUFAs compared to SFAs, and the change in Lachnospiraceae was inversely associated with the change in total cholesterol levels. This is in line with a recent study showing that intake of PUFAs increased the abundance of Lachnospira and Bifidobacterium species in healthy individuals [34]. Findings from an animal study also showed similar results, where Bifidobacterium was negatively correlated with total cholesterol levels in hamsters [35]. Furthermore, oral administration of Bifidobacterium strains has been shown to elicit beneficial health effects, including lowering of total cholesterol levels both in mice [36] and humans [37, 38] and increasing the abundance of Lachnospiraceae [37]. Tindall and colleagues recently showed that members of the Lachnospiraceae family were inversely related to CVD risk factors, including total cholesterol, following a 6-week diet containing walnuts rich in PUFAs [39]. Consistent with these findings, the abundance of Lachnospiraceae was inversely associated with LDL cholesterol in patients with hyperlipidemia treated with rosuvastatin, a third-generation statin drug [40]. After dividing the patients into two groups based on their efficacy for rosuvastatin, those with high efficacy displayed a higher relative abundance of Lachnospiraceae compared to those with lower efficacy [40]. Taken together, the present results and findings from intervention trials might indicate that Lachnospiraceae and Bifidobacterium may act as cholesterol reducing agents.
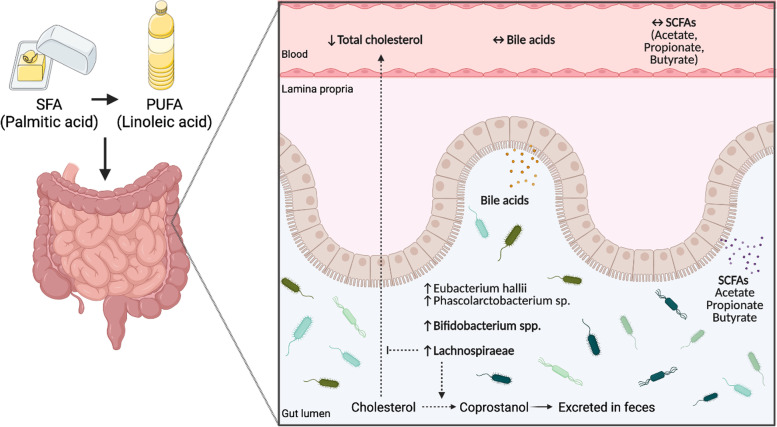
Bile acids are formed from cholesterol in the liver, and increased synthesis and excretion of bile acids may affect cholesterol levels. This is the mechanism for the cholesterol reducing effect of bile acid binding resins. Even though a positive correlation between three bacteria and several bile acids was observed in this study, there was no significant effect on bile acids between interventions; hence, the cholesterol-lowering effect previously observed [1] may not be explained by changes in bile acids. Another explanation for the reduced cholesterol levels might be related to the bacterial conversion of cholesterol to coprostanol, a nonabsorbable sterol [41] (Fig. 6). Approximately 1 g of cholesterol originating from diet and bile enters the colon each day and interferes with colonic bacteria that reduce cholesterol to coprostanol [23]. Coprostanol is poorly absorbed by human intestinal cells and is subsequently excreted via feces [42]. Even though intestinal cholesterol conversion was discovered more than a century ago [41], only limited knowledge about cholesterol metabolism by the gut microbiota exists. In an early study, Wilson found that rats fed palmitic acid (C16:0) or oleic acid (C18:0) had decreased coprostanol formation, while rats fed n6 linoleic acid (C18:2n6) had increased formation of coprostanol from cholesterol [43]. This is in line with the present study, where the exchange of fatty acids was mainly from the SFA palmitic acid to linoleic acid [1]. Linoleic acid is well recognized to decrease cholesterol levels [2, 44, 45], but whether this effect might partially be caused by gut microbiota is still to be elucidated. Other studies also suggest that gut microbes play an important role in regulating serum cholesterol levels. In one study, they showed an inverse relationship between serum cholesterol levels and coprostanol and cholesterol levels in feces [42], and in a recent study by Kenny et al., they identified microbial cholesterol dehydrogenase enzymes and found an association between cholesterol-metabolizing bacteria and reduced serum cholesterol levels [46]. In the present study, an inverse relationship between Lachnospiraceae, Eubacterium hallii and Phascolarctobacterium sp. and total cholesterol levels are demonstrated after replacing intake of SFAs with PUFAs for three days. Interestingly, conversion of cholesterol to coprostanol has previously been shown after only three days [47]; however, the rate of this conversion in humans seems to be highly variable [23, 48]. In line with the present results, most of the cholesterol-reducing bacteria involve members of Eubacterium, but strains of Bifidobacterium and bacteria belonging to the Lachnospiraceae family are also reported to reduce cholesterol to coprostanol [23, 49, 50]. These findings suggest that specific gut bacteria may influence cholesterol metabolism via conversion of cholesterol to coprostanol, but whether this is the case in this study is not known. Therefore, more studies are needed to further elucidate the gut bacterial mechanisms involved in cholesterol regulation.
Fig. 6.
A graphic illustration of the hypothetical mechanisms related to fat quality, gut microbiota, and circulating cholesterol as outlined in the discussion. Replacing SFAs with PUFAs for three days did not affect the levels of bile acids and SCFAs in the circulation. However, gut bacteria may convert cholesterol in the intestine to coprostanol, which is secreted in feces, and hence the circulating levels of cholesterol will decrease. The figure was created with BioRender.com. Abbreviations: PUFAs: polyunsaturated fatty acids, SCFAs: short-chain fatty acids, SFAs: saturated fatty acids
The current findings indicate that fat quality elicits different microbial changes, irrespective of the total fat amount. This is in line with a study by Caesar and colleagues, who showed that mice fed lard and fish oil had different effects on the gut microbiota composition and diversity [21]. Interestingly, the same group reported an interaction between dietary lipids and the gut microbiota in the regulation of hepatic cholesterol metabolism [51]. They showed that in mice fed lard, but not fish oil, the gut microbiota increased hepatic levels of cholesterol and cholesteryl esters. Furthermore, a recent systematic review of randomized controlled trials and observational studies showed that a high fat intake, and in particular a high intake of SFAs, had unfavorable effects on human gut microbiota richness and diversity [52]. In the present study, the fatty acid composition of the study products differed mainly in exchanging palmitic acid with linoleic acid, while n3 fatty acids did not differ between the interventions [1]. There were no correlations between gut bacteria and n6 fatty acids, but there was a negative correlation between EPA and Lachnospiraceae. Selmin et al. found a reduction in Lachnospiraceae after a n6 high-fat diet in mice [30]. Others have shown a positive correlation between circulating levels of docosahexaenoic acid (DHA) (C22:6n3) and Lachnospiraceae [29] and an increase in several butyrate-producing bacteria in the Lachnospiraceae family after intervention with a n3 fatty acid-rich diet in adult males [27]. The Lachnospiraceae family consists of several butyrate-producing bacteria [53]. Even though a significant increase in circulating relative butyrate levels within the PUFA intervention was observed in this study, there were no differences in total SCFAs or butyrate between the interventions or a correlation with Lachnospiraceae. SCFAs are well-known fermentation products from fiber and have been shown to have positive health effects [8]. However, the present study does not indicate that fat and fat quality affect SCFA fermentation.
Taken together, the effect of total fat intake and fat quality on gut microbiota and Lachnospiraceae is not clear. Nevertheless, fat quality may be a promising factor affecting host metabolism by altering the composition and function of the gut microbiota, but robust clinical studies are warranted to investigate this further.
Study strengths and limitations
The randomized controlled crossover design strengthens the findings in the present work, as it corrects for any biological and interindividual differences between participants. This is important when investigating gut microbiota that are known to have large interindividual variation. However, there are some limitations to the study. First, due to the explorative nature of the study, we did not correct for multiple testing. Second, targeted analysis of the gut microbiota, including a panel of 48 bacteria, was performed (in which 40 bacteria were included in statistical analyses), and thus, from this study, it is not known whether other bacteria not included in this panel may impact cholesterol metabolism. The most common bacteria found in the human gut were measured, and specific bacteria associated with cholesterol levels were identified, in line with other studies. However, the Lachnospiraceae family consists of several bacterial species, and there is a need to further investigate which of the bacteria in this family may impact cholesterol levels. Third, even though diet has been shown to affect gut microbiota within one to three days, the short-term duration of the present study (three days per intervention) does not reflect the long-term effects. Fourth, the participants in the present study were normal weight, healthy adults (median age of 28 years); thus, the results cannot be generalized to the population as a whole.
Conclusion and perspectives
In this study, the abundance of the gut bacterial families Lachnospiraceae and Bifidobacterium spp. was increased after replacing SFAs with PUFAs for three days in healthy individuals. Furthermore, the increase in Lachnospiraceae was associated with reduced total cholesterol levels. Whether the reduction in cholesterol levels is linked to linoleic acid-driven coprostanol formation by gut microbiota needs to be elucidated. Future robust studies are needed to investigate whether fat quality may be a promising factor affecting circulating cholesterol levels in the host by altering the composition and function of the gut microbiota. Dietary manipulation of the gut microbiota might be important for lipid-lowering prevention and treatment in the future.
Acknowledgements
We are grateful to all participants in the study and to bioengineer Ellen Raael for her contributions to the present study.
Abbreviations
- A
Actinobacteria
- B
Bacteroidetes
- CA
Cholic acid
- CDCA
Chenodeoxycholic acid
- CVD
Cardiovascular diseases
- DCA
Deoxycholic acid
- DHA
Docosahexaenoic acid
- EPA
Eicosapentaenoic acid
- F
Firmicutes
- FFM
Fat free mass
- GCA
Glycocholic acid
- GDCA
Glycodeoxycholic acid
- GCDCA
Glycochenodeoxycholic acid
- HbA1c
Hemoglobin A1c
- hsCRP
High sensitivity C reactive protein
- n
Number
- n3
Omega 3
- n6
Omega 6
- NEFA
Nonesterified fatty acid
- ns
Nonsignificant
- P
Proteobacteria
- PUFA
Polyunsaturated fatty acid
- RCT
Randomized controlled trial
- SCFA
Short-chain fatty acid
- SFA
Saturated fatty acid
- T
Tenericutes
- TCA
Taurocholic acid
- TDCA
Taurodeoxycholic acid
- TCDCA
Taurochenodeoxycholic acid
Authors’ contributions
VHTH, LG, KBH, SMU and MCWM formulated the research questions and designed the study; VHTH, LG and MCWM conducted the study; MGB provided study products; NB conducted the bile acid analyses; VHTH and LG performed statistical analyses and prepared the figures; VHTH, LG, IR, NB, MGB, TG, KR, KBH, SMU and MCWM interpreted the data; VHTH and LG drafted the article; VHTH, LG and MCWM had primary responsibility for the final content; all authors read and approved the final version of the manuscript.
Funding
This research received no specific grant from any funding agency or not-for-profit sector. This work was supported by OsloMet, UiO and Mills AS. OsloMet and UiO supported the study with direct financial support. Mills AS supported the study with direct financial support and with the provision of study products.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Regional Committees for Medical and Health Research Ethics (2018/104). Written informed consent was obtained from all subjects. The study was registered at Clinical Trials (https://clinicaltrials.gov/, registration identification number: NCT03658681).
Consent for publication
Not applicable.
Competing interests
LG, NB, TG and IR report nothing to declare. VHTH has been employed at Mills AS and reports grants from Mills AS. She does not own any stocks in the company, and the work performed in this paper was done after she left the company. MGB is employed at Mills AS. She does not own any stocks in the company. She did not have a role in the design of the study and analysis of the data and was not involved in the statistical analysis. KR reports personal fees from MedXplore, Amgen, Mills AS, The Norwegian Medical Association, The Norwegian Directorate of Health, Sanofi, Takeda, Chiesi, Bayer and MSD, outside the submitted work. KBH reports grants from Tine SA, Mills AS, Olympic Seafood, Amgen, Sanofi, Kaneka and personal fees from Amgen, Sanofi, Pronova, outside the submitted work. SMU has received research grants from Tine DA, Mills AS, and Olympic Seafood, none of which are related to the content of this manuscript. MCWM is involved in projects with research grants from Tine SA and Olympic Seafood and has received research funds from Mills AS, none of which are related to the content of this manuscript.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Vibeke H. Telle-Hansen and Line Gaundal are shared first authorship.
References
- 1.Gaundal L, Myhrstad MCW, Leder L, Byfuglien MG, Gjovaag T, Rud I, et al. Beneficial effect on serum cholesterol levels, but not glycaemic regulation, after replacing SFA with PUFA for 3 d: a randomised crossover trial. Br J Nutr. 2021;125(8):915–925. doi: 10.1017/S0007114520003402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ulven SM, Leder L, Elind E, Ottestad I, Christensen JJ, Telle-Hansen VH, et al. Exchanging a few commercial, regularly consumed food items with improved fat quality reduces total cholesterol and LDL-cholesterol: a double-blind, randomised controlled trial. Br J Nutr. 2016;116(8):1383–1393. doi: 10.1017/S0007114516003445. [DOI] [PubMed] [Google Scholar]
- 3.Schwab U, Lauritzen L, Tholstrup T, Haldorssoni T, Riserus U, Uusitupa M, et al. Effect of the amount and type of dietary fat on cardiometabolic risk factors and risk of developing type 2 diabetes, cardiovascular diseases, and cancer: a systematic review. Food Nutr Res. 2014;58:25–45. doi: 10.3402/fnr.v58.25145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mozaffarian D, Micha R, Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2010;7(3):e1000252. doi: 10.1371/journal.pmed.1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lovegrove JA. Dietary dilemmas over fats and cardiometabolic risk. Proc Nutr Soc. 2020;79(1):11–21. doi: 10.1017/S0029665119000983. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez MAG, Canfora EE, Jocken JWE, Blaak EE. The Short-Chain Fatty Acid Acetate in Body Weight Control and Insulin Sensitivity. Nutrients. 2019;11(8):1943. doi: 10.3390/nu11081943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma S, Tripathi P. Gut microbiome and type 2 diabetes: where we are and where to go? J Nutr Biochem. 2019;63:101–108. doi: 10.1016/j.jnutbio.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. 2015;11(10):577–591. doi: 10.1038/nrendo.2015.128. [DOI] [PubMed] [Google Scholar]
- 9.Allin KH, Nielsen T, Pedersen O. Mechanisms in endocrinology: Gut microbiota in patients with type 2 diabetes mellitus. Eur J Endocrinol. 2015;172(4):R167–R177. doi: 10.1530/EJE-14-0874. [DOI] [PubMed] [Google Scholar]
- 10.Asnicar F, Berry SE, Valdes AM, Nguyen LH, Piccinno G, Drew DA, et al. Microbiome connections with host metabolism and habitual diet from 1,098 deeply phenotyped individuals. Nat Med. 2021;27(2):321–332. doi: 10.1038/s41591-020-01183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansen NW, Sams A. The Microbiotic Highway to Health-New Perspective on Food Structure, Gut Microbiota, and Host Inflammation. Nutrients. 2018;10(11):1590. doi: 10.3390/nu10111590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107(33):14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kovatcheva-Datchary P, Nilsson A, Akrami R, Lee YS, De Vadder F, Arora T, et al. Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metab. 2015;22(6):971–982. doi: 10.1016/j.cmet.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science (New York, NY) 2011;334(6052):105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1(6):6ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schoeler M, Caesar R. Dietary lipids, gut microbiota and lipid metabolism. Rev Endocr Metab Disord. 2019;20(4):461–472. doi: 10.1007/s11154-019-09512-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lappi J, Salojärvi J, Kolehmainen M, Mykkänen H, Poutanen K, de Vos WM, et al. Intake of Whole-Grain and Fiber-Rich Rye Bread Versus Refined Wheat Bread Does Not Differentiate Intestinal Microbiota Composition in Finnish Adults with Metabolic Syndrome. J Nutr. 2013;143(5):648–655. doi: 10.3945/jn.112.172668. [DOI] [PubMed] [Google Scholar]
- 19.Candido FG, Valente FX, Grzeskowiak LM, Moreira APB, Rocha D, Alfenas RCG. Impact of dietary fat on gut microbiota and low-grade systemic inflammation: mechanisms and clinical implications on obesity. Int J Food Sci Nutr. 2018;69(2):125–143. doi: 10.1080/09637486.2017.1343286. [DOI] [PubMed] [Google Scholar]
- 20.Simoes CD, Maukonen J, Kaprio J, Rissanen A, Pietilainen KH, Saarela M. Habitual dietary intake is associated with stool microbiota composition in monozygotic twins. J Nutr. 2013;143(4):417–423. doi: 10.3945/jn.112.166322. [DOI] [PubMed] [Google Scholar]
- 21.Caesar R, Tremaroli V, Kovatcheva-Datchary P, Cani PD, Backhed F. Crosstalk between Gut Microbiota and Dietary Lipids Aggravates WAT Inflammation through TLR Signaling. Cell Metab. 2015;22(4):658–668. doi: 10.1016/j.cmet.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489(7415):242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 23.Kriaa A, Bourgin M, Potiron A, Mkaouar H, Jablaoui A, Gérard P, et al. Microbial impact on cholesterol and bile acid metabolism: current status and future prospects. J Lipid Res. 2019;60(2):323–332. doi: 10.1194/jlr.R088989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. BMJ (Clinical research ed) 2018;361:k2179. doi: 10.1136/bmj.k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Wit N, Derrien M, Bosch-Vermeulen H, Oosterink E, Keshtkar S, Duval C, et al. Saturated fat stimulates obesity and hepatic steatosis and affects gut microbiota composition by an enhanced overflow of dietary fat to the distal intestine. Am J Physiol Gastrointest Liver Physiol. 2012;303(5):G589–G599. doi: 10.1152/ajpgi.00488.2011. [DOI] [PubMed] [Google Scholar]
- 26.Mujico JR, Baccan GC, Gheorghe A, Díaz LE, Marcos A. Changes in gut microbiota due to supplemented fatty acids in diet-induced obese mice. Br J Nutr. 2013;110(4):711–720. doi: 10.1017/S0007114512005612. [DOI] [PubMed] [Google Scholar]
- 27.Noriega BS, Sanchez-Gonzalez MA, Salyakina D, Coffman J. Understanding the Impact of Omega-3 Rich Diet on the Gut Microbiota. Case Rep Med. 2016;2016:3089303. doi: 10.1155/2016/3089303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costantini L, Molinari R, Farinon B, Merendino N. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int J Mol Sci. 2017;18(12):2645. doi: 10.3390/ijms18122645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menni C, Zierer J, Pallister T, Jackson MA, Long T, Mohney RP, et al. Omega-3 fatty acids correlate with gut microbiome diversity and production of N-carbamylglutamate in middle aged and elderly women. Sci Rep. 2017;7(1):11079. doi: 10.1038/s41598-017-10382-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Selmin OI, Papoutsis AJ, Hazan S, Smith C, Greenfield N, Donovan MG, et al. n-6 High Fat Diet Induces Gut Microbiome Dysbiosis and Colonic Inflammation. Int J Mol Sci. 2021;22(13):6919. doi: 10.3390/ijms22136919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiriyama Y, Nochi H. Physiological Role of Bile Acids Modified by the Gut Microbiome. Microorganisms. 2021;10(1):68. doi: 10.3390/microorganisms10010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Callender C, Attaye I, Nieuwdorp M. The Interaction between the Gut Microbiome and Bile Acids in Cardiometabolic Diseases. Metabolites. 2022;12(1):65. doi: 10.3390/metabo12010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casen C, Vebo HC, Sekelja M, Hegge FT, Karlsson MK, Ciemniejewska E, et al. Deviations in human gut microbiota: a novel diagnostic test for determining dysbiosis in patients with IBS or IBD. Aliment Pharmacol Ther. 2015;42(1):71–83. doi: 10.1111/apt.13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watson H, Mitra S, Croden FC, Taylor M, Wood HM, Perry SL, et al. A randomised trial of the effect of omega-3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut. 2018;67(11):1974–1983. doi: 10.1136/gutjnl-2017-314968. [DOI] [PubMed] [Google Scholar]
- 35.Tong A-J, Hu R-K, Wu L-X, Lv X-C, Li X, Zhao L-N, et al. Ganoderma polysaccharide and chitosan synergistically ameliorate lipid metabolic disorders and modulate gut microbiota composition in high fat diet-fed golden hamsters. J Food Biochem. 2020;44(1):e13109. doi: 10.1111/jfbc.13109. [DOI] [PubMed] [Google Scholar]
- 36.Bordoni A, Amaretti A, Leonardi A, Boschetti E, Danesi F, Matteuzzi D, et al. Cholesterol-lowering probiotics: in vitro selection and in vivo testing of bifidobacteria. Appl Microbiol Biotechnol. 2013;97(18):8273–8281. doi: 10.1007/s00253-013-5088-2. [DOI] [PubMed] [Google Scholar]
- 37.Wang K, Yu X, Li Y, Guo Y, Ge L, Pu F, et al. Bifidobacterium bifidum TMC3115 Can Characteristically Influence Glucose and Lipid Profile and Intestinal Microbiota in the Middle-Aged and Elderly. Probiotics Antimicrob Proteins. 2019;11(4):1182–1194. doi: 10.1007/s12602-018-9441-8. [DOI] [PubMed] [Google Scholar]
- 38.Guo Z, Liu XM, Zhang QX, Shen Z, Tian FW, Zhang H, et al. Influence of consumption of probiotics on the plasma lipid profile: A meta-analysis of randomised controlled trials. Nutr Metab Cardiovasc Dis. 2011;21(11):844–850. doi: 10.1016/j.numecd.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 39.Tindall AM, McLimans CJ, Petersen KS, Kris-Etherton PM, Lamendella R. Walnuts and Vegetable Oils Containing Oleic Acid Differentially Affect the Gut Microbiota and Associations with Cardiovascular Risk Factors: Follow-up of a Randomized, Controlled, Feeding Trial in Adults at Risk for Cardiovascular Disease. J Nutr. 2020;150(4):806–817. doi: 10.1093/jn/nxz289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y, Song X, Zhou H, Zhou X, Xia Y, Dong X, et al. Gut Microbiome Associates With Lipid-Lowering Effect of Rosuvastatin in Vivo. Front Microbiol. 2018;9:530. doi: 10.3389/fmicb.2018.00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Juste C, Gerard P. Cholesterol-to-Coprostanol Conversion by the Gut Microbiota: What We Know, Suspect, and Ignore. Microorganisms. 2021;9(9):1881. doi: 10.3390/microorganisms9091881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sekimoto H, Shimada O, Makanishi M, Nakano T, Katayama O. Interrelationship between serum and fecal sterols. Jpn J Med. 1983;22(1):14–20. doi: 10.2169/internalmedicine1962.22.14. [DOI] [PubMed] [Google Scholar]
- 43.Wilson JD. The effect of dietary fatty acids on coprostanol excretion by the rat. J Lipid Res. 1961;2(4):7. doi: 10.1016/S0022-2275(20)40478-X. [DOI] [Google Scholar]
- 44.Kris-Etherton PM, Yu S. Individual fatty acid effects on plasma lipids and lipoproteins: human studies. Am J Clin Nutr. 1997;65(5 Suppl):1628S–S1644. doi: 10.1093/ajcn/65.5.1628S. [DOI] [PubMed] [Google Scholar]
- 45.Froyen E, Burns-Whitmore B. The Effects of Linoleic Acid Consumption on Lipid Risk Markers for Cardiovascular Disease in Healthy Individuals: A Review of Human Intervention Trials. Nutrients. 2020;12(8):2329. doi: 10.3390/nu12082329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kenny DJ, Plichta DR, Shungin D, Koppel N, Hall AB, Fu B, et al. Cholesterol Metabolism by Uncultured Human Gut Bacteria Influences Host Cholesterol Level. Cell Host Microbe. 2020;28(2):245–57 e6. doi: 10.1016/j.chom.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gérard P, Lepercq P, Leclerc M, Gavini F, Raibaud P, Juste C. Bacteroides sp. Strain D8, the First Cholesterol-Reducing Bacterium Isolated from Human Feces. Appl Environ Microbiol. 2007;73(18):5742–9. doi: 10.1128/AEM.02806-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Veiga P, Juste C, Lepercq P, Saunier K, Béguet F, Gérard P. Correlation between faecal microbial community structure and cholesterol-to-coprostanol conversion in the human gut. FEMS Microbiol Lett. 2005;242(1):81–86. doi: 10.1016/j.femsle.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 49.Antharam VC, McEwen DC, Garrett TJ, Dossey AT, Li EC, Kozlov AN, et al. An Integrated Metabolomic and Microbiome Analysis Identified Specific Gut Microbiota Associated with Fecal Cholesterol and Coprostanol in Clostridium difficile Infection. PLoS ONE. 2016;11(2):e0148824. doi: 10.1371/journal.pone.0148824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crowther JS, Drasar BS, Goddard P, Hill MJ, Johnson K. The effect of a chemically defined diet on the faecal flora and faecal steroid concentration. Gut. 1973;14(10):790–793. doi: 10.1136/gut.14.10.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caesar R, Nygren H, Orešič M, Bäckhed F. Interaction between dietary lipids and gut microbiota regulates hepatic cholesterol metabolism. J Lipid Res. 2016;57(3):474–481. doi: 10.1194/jlr.M065847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wolters M, Ahrens J, Romaní-Pérez M, Watkins C, Sanz Y, Benítez-Páez A, et al. Dietary fat, the gut microbiota, and metabolic health – A systematic review conducted within the MyNewGut project. Clin Nutr. 2019;38(6):2504–2520. doi: 10.1016/j.clnu.2018.12.024. [DOI] [PubMed] [Google Scholar]
- 53.Vacca M, Celano G, Calabrese FM, Portincasa P, Gobbetti M, De Angelis M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms. 2020;8(4):573. doi: 10.3390/microorganisms8040573. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.