Scheme 2.
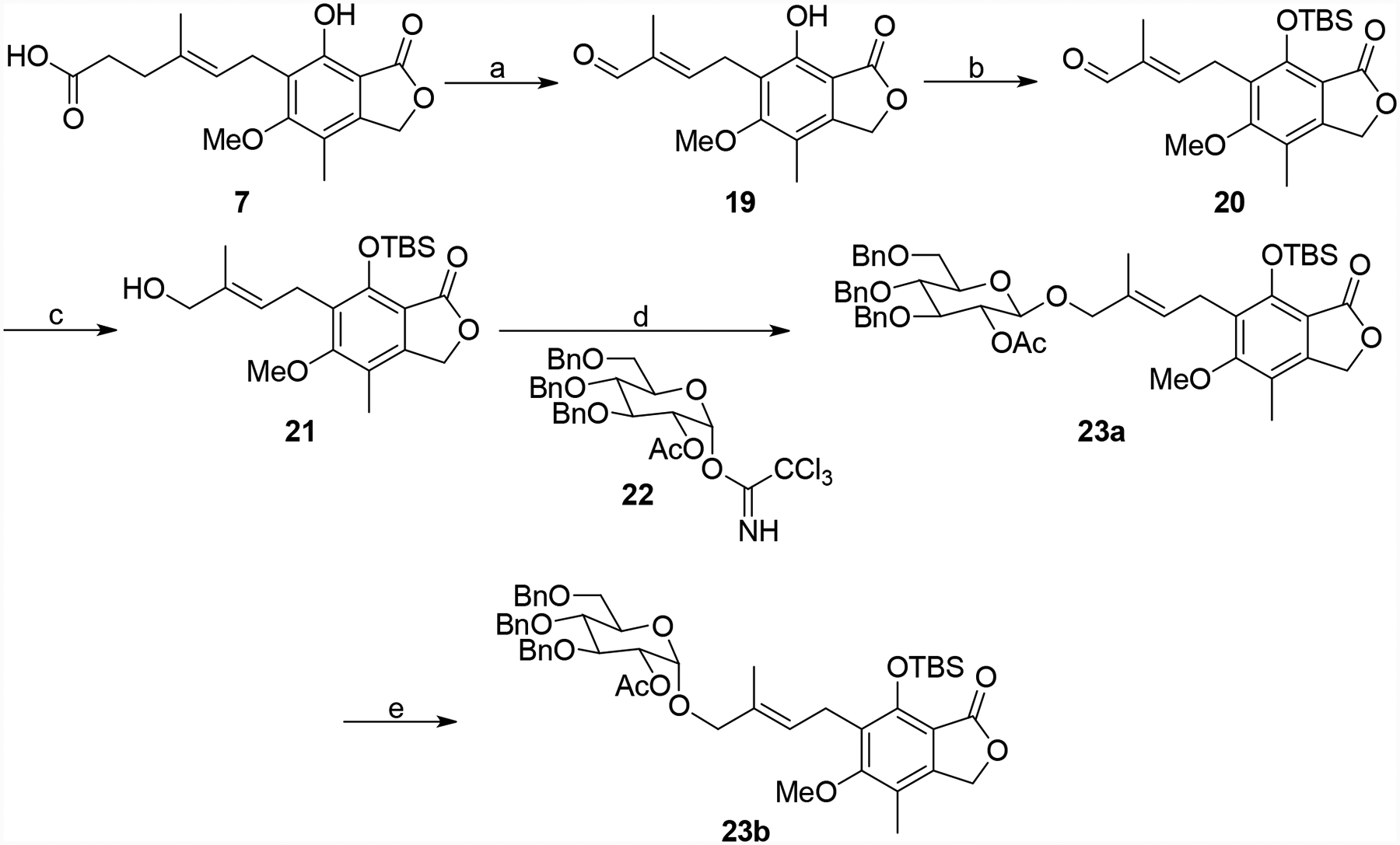
Synthesis of intermediates 23a and 23b. Reagents and conditions: (a) i) OsO4, NMO, NaIO4, THF/H2O, rt, 1.5 h. ii) PPh3=C(CH3)CHO, benzene, reflux, 24 h, 77%. (b) TBSCl, imidazole, DCM, rt, 16 h, 71%. (c) NaBH, MeOH, 0 °4 C to rt, 1 h, 92%. (d) 22, BF3•OEt2, DCM, −78 °C for 0.5 h then 0 °C for 0.5 h, 88%. (e) TiCl4, DCM, −78°C for 10 min then 0 °C for 0.5 h, 99%.
