Scheme 4.
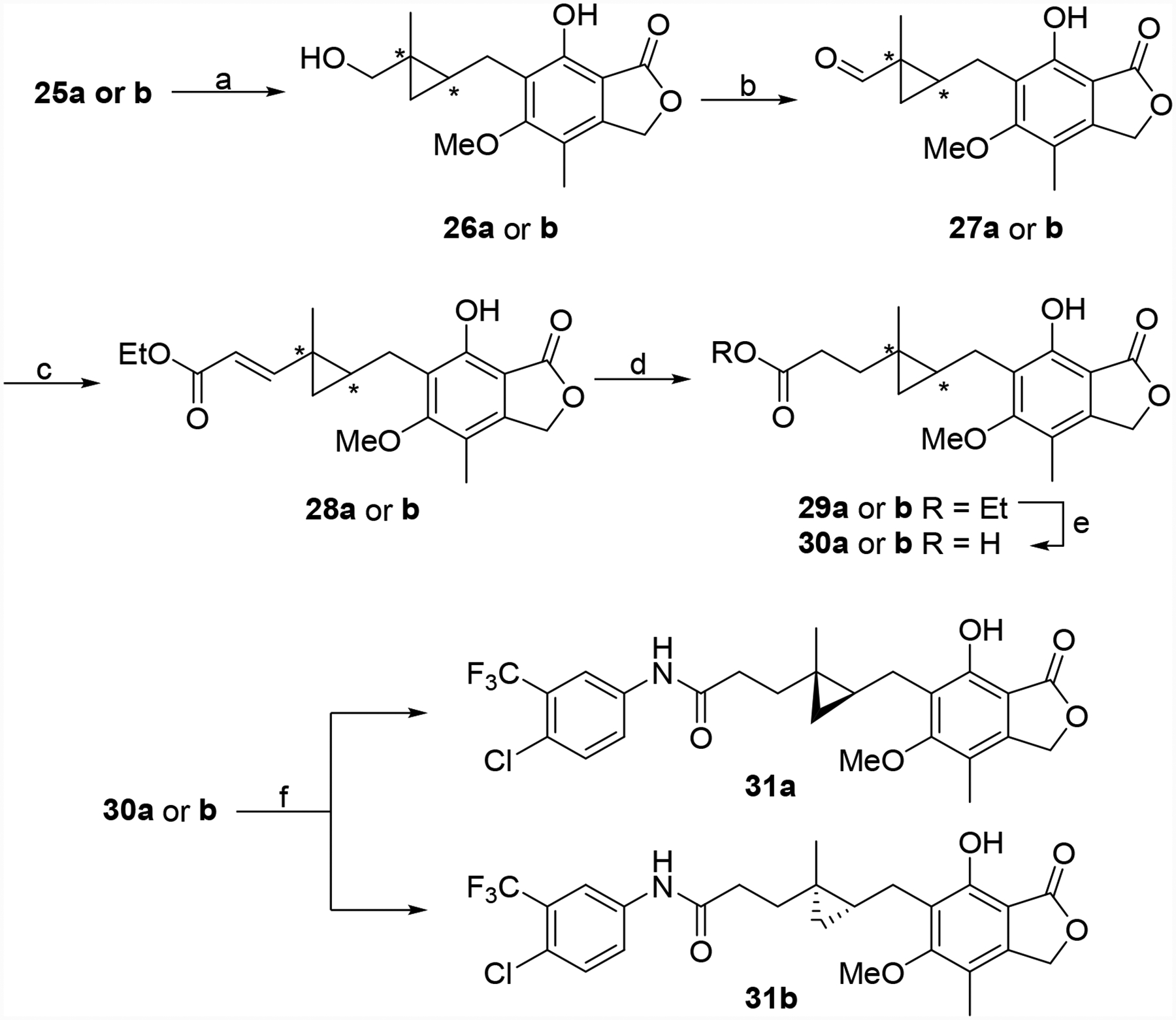
Synthesis of 31a and 31b. Reagents and conditions: (a) i) Tf2O, pyridine, DCM, −20 °C to rt; ii) DMF, pyridine, H2O, 120 °C, 10 min, 81% and 93%. (b) DMP, DCM, rt, 0.5 h, 99%, and 83%. (c) triethyl phosphonoacetate, NaH, benzene, rt, 1 h, 57% and 51% (d) CoCl2, NaBH4, MeOH/DMF, rt, 0.5 h, 62% and 87% (e) LiOH, THF/H2O, rt, 3 h, 86% and 85%. (f) 4-chloro-3-(trifluoromethyl)aniline, EDC•HCl, HOAt, DMF, rt, 16 h, 81% and 71%.
