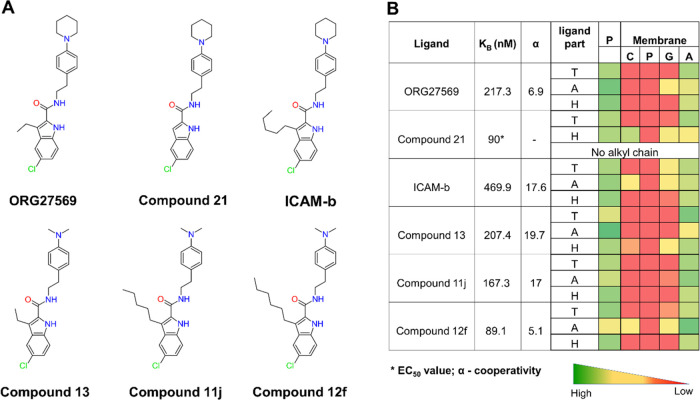
Figure 8.
ORG27569’s analogs show distinct contacts with the membrane with varying alkyl chain lengths. (A) 2D structures of ORG27569 and its analogs. (B) Binding affinity of the ligands to the allosteric site (as either the equilibrium dissociation constant KB or the EC50 value), the binding cooperativity factor (α), and the extent of contact with the protein and lipids of the studied ORG27569 analogs. The heatmap shows the percentage occupancy (the fraction of the simulation time during which the given ligand is within 4 Å of the protein residue or membrane lipids) was calculated as the extent of contact the ligand atoms make with the various lipid components throughout the simulation time. The longer alkyl chains at C-3 positions of the analogs such as Compounds 11j, Compound 12f, and ICAM-b appear to be mostly in contact with the membrane lipids. Ligand parts: T—tail (piperidinyl-benzyl rings), A—alkyl chain at the C-3 position, and H—head (indole ring); P—protein residues; and Membrane: C—Choline, P—Phosphate, G—Glyceryl, and A—Alkyl.